Spatial coupling between DNA replication and mismatch repair in Caulobacter crescentus
- PMID: 33677508
- PMCID: PMC8034640
- DOI: 10.1093/nar/gkab112
Spatial coupling between DNA replication and mismatch repair in Caulobacter crescentus
Abstract
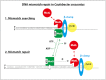
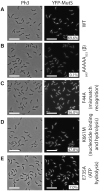
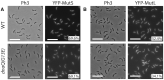
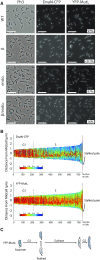
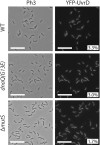
The DNA mismatch repair (MMR) process detects and corrects replication errors in organisms ranging from bacteria to humans. In most bacteria, it is initiated by MutS detecting mismatches and MutL nicking the mismatch-containing DNA strand. Here, we show that MMR reduces the appearance of rifampicin resistances more than a 100-fold in the Caulobacter crescentus Alphaproteobacterium. Using fluorescently-tagged and functional MutS and MutL proteins, live cell microscopy experiments showed that MutS is usually associated with the replisome during the whole S-phase of the C. crescentus cell cycle, while MutL molecules may display a more dynamic association with the replisome. Thus, MMR components appear to use a 1D-scanning mode to search for rare mismatches, although the spatial association between MutS and the replisome is dispensible under standard growth conditions. Conversely, the spatial association of MutL with the replisome appears as critical for MMR in C. crescentus, suggesting a model where the β-sliding clamp licences the endonuclease activity of MutL right behind the replication fork where mismatches are generated. The spatial association between MMR and replisome components may also play a role in speeding up MMR and/or in recognizing which strand needs to be repaired in a variety of Alphaproteobacteria.
© The Author(s) 2021. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures



Similar articles
-
Recurrent mismatch binding by MutS mobile clamps on DNA localizes repair complexes nearby.Proc Natl Acad Sci U S A. 2020 Jul 28;117(30):17775-17784. doi: 10.1073/pnas.1918517117. Epub 2020 Jul 15. Proc Natl Acad Sci U S A. 2020. PMID: 32669440 Free PMC article.
-
Stoichiometry of MutS and MutL at unrepaired mismatches in vivo suggests a mechanism of repair.Nucleic Acids Res. 2012 May;40(9):3929-38. doi: 10.1093/nar/gkr1298. Epub 2012 Jan 12. Nucleic Acids Res. 2012. PMID: 22241777 Free PMC article.
-
DnaN clamp zones provide a platform for spatiotemporal coupling of mismatch detection to DNA replication.Mol Microbiol. 2013 Feb;87(3):553-68. doi: 10.1111/mmi.12115. Epub 2012 Dec 11. Mol Microbiol. 2013. PMID: 23228104 Free PMC article.
-
DNA mismatch repair and mutation avoidance pathways.J Cell Physiol. 2002 Apr;191(1):28-41. doi: 10.1002/jcp.10077. J Cell Physiol. 2002. PMID: 11920679 Review.
-
Mismatch repair in Gram-positive bacteria.Res Microbiol. 2016 Jan;167(1):4-12. doi: 10.1016/j.resmic.2015.08.006. Epub 2015 Sep 3. Res Microbiol. 2016. PMID: 26343983 Review.
Cited by
-
The repressor PrtR1 and the global H-NS-like regulators MvaT and MvaV enable the fine-tuning of R-tailocin expression in Pseudomonas protegens.BMC Microbiol. 2025 May 11;25(1):286. doi: 10.1186/s12866-025-03983-9. BMC Microbiol. 2025. PMID: 40350448 Free PMC article.
-
Mechanistic insight into the repair of C8-linked pyrrolobenzodiazepine monomer-mediated DNA damage.RSC Med Chem. 2022 Oct 18;13(12):1621-1633. doi: 10.1039/d2md00194b. eCollection 2022 Dec 14. RSC Med Chem. 2022. PMID: 36561066 Free PMC article.
-
Bacterial DNA excision repair pathways.Nat Rev Microbiol. 2022 Aug;20(8):465-477. doi: 10.1038/s41579-022-00694-0. Epub 2022 Feb 24. Nat Rev Microbiol. 2022. PMID: 35210609 Free PMC article. Review.
-
The structure of the MutL-CTD:processivity-clamp complex provides insight regarding strand discrimination in non-methyl-directed DNA mismatch repair.Nucleic Acids Res. 2025 Feb 8;53(4):gkaf094. doi: 10.1093/nar/gkaf094. Nucleic Acids Res. 2025. PMID: 39988319 Free PMC article.
References
-
- Iyer R.R., Pluciennik A., Burdett V., Modrich P.L.. DNA mismatch repair: functions and mechanisms. Chem. Rev. 2006; 106:302–323. - PubMed
-
- Fishel R., Lescoe M.K., Rao M.R., Copeland N.G., Jenkins N.A., Garber J., Kane M., Kolodner R.. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell. 1993; 75:1027–1038. - PubMed
-
- Li G.M. Mechanisms and functions of DNA mismatch repair. Cell Res. 2008; 18:85–98. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

