Diminished Cortical Excitation and Elevated Inhibition During Perceptual Impairments in a Mouse Model of Autism
- PMID: 33677512
- PMCID: PMC8525192
- DOI: 10.1093/cercor/bhab025
Diminished Cortical Excitation and Elevated Inhibition During Perceptual Impairments in a Mouse Model of Autism
Abstract
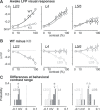
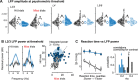
Sensory impairments are a core feature of autism spectrum disorder (ASD). These impairments affect visual perception and have been hypothesized to arise from imbalances in cortical excitatory and inhibitory activity. There is conflicting evidence for this hypothesis from several recent studies of transgenic mouse models of ASD; crucially, none have measured activity from identified excitatory and inhibitory neurons during simultaneous impairments of sensory perception. Here, we directly recorded putative excitatory and inhibitory population spiking in primary visual cortex (V1) while simultaneously measuring visual perceptual behavior in CNTNAP2-/- knockout (KO) mice. We observed quantitative impairments in the speed, accuracy, and contrast sensitivity of visual perception in KO mice. During these perceptual impairments, stimuli evoked more firing of inhibitory neurons and less firing of excitatory neurons, with reduced neural sensitivity to contrast. In addition, pervasive 3-10 Hz oscillations in superficial cortical layers 2/3 (L2/3) of KO mice degraded predictions of behavioral performance from neural activity. Our findings show that perceptual deficits relevant to ASD may be associated with elevated cortical inhibitory activity along with diminished and aberrant excitatory population activity in L2/3, a major source of feedforward projections to higher cortical regions.
Keywords: autism; excitation; inhibition; mouse; perception; visual cortex.
© The Author(s) 2021. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures



References
-
- American Psychiatric Association. D-TF . 2013. Diagnostic and statistical manual of mental disorders : DSM-5. 5th ed. Washington (DC): American Psychiatric Association.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

