Low antileishmanial drug exposure in HIV-positive visceral leishmaniasis patients on antiretrovirals: an Ethiopian cohort study
- PMID: 33677546
- PMCID: PMC8050768
- DOI: 10.1093/jac/dkab013
Low antileishmanial drug exposure in HIV-positive visceral leishmaniasis patients on antiretrovirals: an Ethiopian cohort study
Abstract
Background: Despite high HIV co-infection prevalence in Ethiopian visceral leishmaniasis (VL) patients, the adequacy of antileishmanial drug exposure in this population and effect of HIV-VL co-morbidity on pharmacokinetics of antileishmanial and antiretroviral (ARV) drugs is still unknown.
Methods: HIV-VL co-infected patients received the recommended liposomal amphotericin B (LAmB) monotherapy (total dose 40 mg/kg over 24 days) or combination therapy of LAmB (total dose 30 mg/kg over 11 days) plus 28 days 100 mg/day miltefosine, with possibility to extend treatment for another cycle. Miltefosine, total amphotericin B and ARV concentrations were determined in dried blood spots or plasma using LC-MS/MS.
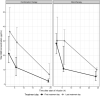
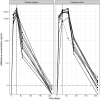
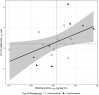
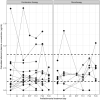
Results: Median (IQR) amphotericin B Cmax on Day 1 was 24.6 μg/mL (17.0-34.9 μg/mL), which increased to 40.9 (25.4-53.1) and 33.2 (29.0-46.6) μg/mL on the last day of combination and monotherapy, respectively. Day 28 miltefosine concentration was 18.7 (15.4-22.5) μg/mL. Miltefosine exposure correlated with amphotericin B accumulation. ARV concentrations were generally stable during antileishmanial treatment, although efavirenz Cmin was below the 1 μg/mL therapeutic target for many patients.
Conclusions: This study demonstrates that antileishmanial drug exposure was low in this cohort of HIV co-infected VL patients. Amphotericin B Cmax was 2-fold lower than previously observed in non-VL patients. Miltefosine exposure in HIV-VL co-infected patients was 35% lower compared with adult VL patients in Eastern Africa, only partially explained by a 19% lower dose, possibly warranting a dose adjustment. Adequate drug exposure in these HIV-VL co-infected patients is especially important given the high proportion of relapses.
© The Author(s) 2021. Published by Oxford University Press on behalf of the British Society for Antimicrobial Chemotherapy.
Figures
Similar articles
-
The initial effectiveness of liposomal amphotericin B (AmBisome) and miltefosine combination for treatment of visceral leishmaniasis in HIV co-infected patients in Ethiopia: A retrospective cohort study.PLoS Negl Trop Dis. 2018 May 25;12(5):e0006527. doi: 10.1371/journal.pntd.0006527. eCollection 2018 May. PLoS Negl Trop Dis. 2018. PMID: 29799869 Free PMC article.
-
A randomized trial of AmBisome monotherapy and AmBisome and miltefosine combination to treat visceral leishmaniasis in HIV co-infected patients in Ethiopia.PLoS Negl Trop Dis. 2019 Jan 17;13(1):e0006988. doi: 10.1371/journal.pntd.0006988. eCollection 2019 Jan. PLoS Negl Trop Dis. 2019. PMID: 30653490 Free PMC article. Clinical Trial.
-
Visceral Leishmaniasis and HIV coinfection in East Africa.PLoS Negl Trop Dis. 2014 Jun 26;8(6):e2869. doi: 10.1371/journal.pntd.0002869. eCollection 2014 Jun. PLoS Negl Trop Dis. 2014. PMID: 24968313 Free PMC article. Review.
-
Multiple relapses of visceral leishmaniasis in a patient with HIV in India: a treatment challenge.Int J Infect Dis. 2014 Aug;25:204-6. doi: 10.1016/j.ijid.2014.02.015. Epub 2014 Jun 10. Int J Infect Dis. 2014. PMID: 24927662
-
Chemotherapeutics of visceral leishmaniasis: present and future developments.Parasitology. 2018 Apr;145(4):481-489. doi: 10.1017/S0031182017002116. Epub 2017 Dec 7. Parasitology. 2018. PMID: 29215329 Free PMC article. Review.
Cited by
-
The spleen is the graveyard of CD4+ cells in patients with immunological failure of visceral leishmaniasis and AIDS.Parasit Vectors. 2024 Mar 15;17(1):132. doi: 10.1186/s13071-024-06151-6. Parasit Vectors. 2024. PMID: 38491526 Free PMC article.
-
Precision Medicine in Control of Visceral Leishmaniasis Caused by L. donovani.Front Cell Infect Microbiol. 2021 Nov 9;11:707619. doi: 10.3389/fcimb.2021.707619. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34858865 Free PMC article. Review.
-
Pharmacokinetics of Antiretroviral Drugs in Older People Living with HIV, Part II: Drugs Licensed Before 2005.Clin Pharmacokinet. 2024 Dec;63(12):1655-1666. doi: 10.1007/s40262-024-01441-9. Epub 2024 Nov 14. Clin Pharmacokinet. 2024. PMID: 39542985
-
Low Plasma Lipids Are Associated with Relapsing and Lethal Visceral Leishmaniasis in HIV-Infected Patients.Pathogens. 2024 May 25;13(6):450. doi: 10.3390/pathogens13060450. Pathogens. 2024. PMID: 38921748 Free PMC article.
References
-
- Ritmeijer K, Veeken H, Melaku Y. et al. Ethiopian visceral leishmaniasis: generic and proprietary sodium stibogluconate are equivalent; HIV co-infected patients have a poor outcome. Trans R Soc Trop Med Hyg 2001; 95: 668–72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

