Oxygenation as a driving factor in epithelial differentiation at the air-liquid interface
- PMID: 33677549
- PMCID: PMC7965686
- DOI: 10.1093/intbio/zyab002
Oxygenation as a driving factor in epithelial differentiation at the air-liquid interface
Abstract
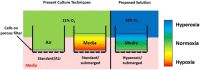
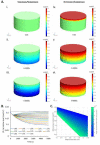
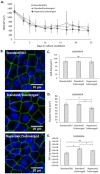
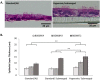
Culture at the air-liquid interface is broadly accepted as necessary for differentiation of cultured epithelial cells towards an in vivo-like phenotype. However, air-liquid interface cultures are expensive, laborious and challenging to scale for increased throughput applications. Deconstructing the microenvironmental parameters that drive these differentiation processes could circumvent these limitations, and here we hypothesize that reduced oxygenation due to diffusion limitations in liquid media limits differentiation in submerged cultures; and that this phenotype can be rescued by recreating normoxic conditions at the epithelial monolayer, even under submerged conditions. Guided by computational models, hyperoxygenation of atmospheric conditions was applied to manipulate oxygenation at the monolayer surface. The impact of this rescue condition was confirmed by assessing protein expression of hypoxia-sensitive markers. Differentiation of primary human bronchial epithelial cells isolated from healthy patients was then assessed in air-liquid interface, submerged and hyperoxygenated submerged culture conditions. Markers of differentiation, including epithelial layer thickness, tight junction formation, ciliated surface area and functional capacity for mucociliary clearance, were assessed and found to improve significantly in hyperoxygenated submerged cultures, beyond standard air-liquid interface or submerged culture conditions. These results demonstrate that an air-liquid interface is not necessary to produce highly differentiated epithelial structures, and that increased availability of oxygen and nutrient media can be leveraged as important strategies to improve epithelial differentiation for applications in respiratory toxicology and therapeutic development.
Keywords: air–liquid interface; epithelium; microenvironment; oxygenation.
© The Author(s) 2021. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permission@oup.com.
Figures


References
-
- Jiang D, Schaefer N, Chu HW. In: Alper S, Janssen WJ (eds.). Air-liquid interface culture of human and mouse airway epithelial cells. Lung Innate Immunity and Inflammation: Methods and Protocols. New York, Springer, 2018, 91–109. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

