Cancer Immunotherapy Update: FDA-Approved Checkpoint Inhibitors and Companion Diagnostics
- PMID: 33677681
- PMCID: PMC7937597
- DOI: 10.1208/s12248-021-00574-0
Cancer Immunotherapy Update: FDA-Approved Checkpoint Inhibitors and Companion Diagnostics
Abstract
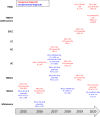
Immune checkpoint inhibitors (ICIs) are considered a new standard-of-care across many cancer indications. This review provides an update on ICIs approved by the Food and Drug Administration (FDA), with focus on monoclonal antibodies that target the programmed cell death 1 (PD-1) or its ligand, PD-1 ligand 1 (PD-L1), including information on their clinical indications and associated companion diagnostics. The information is further discussed with strategies for identifying predictive biomarkers to guide the clinical use of PD-1/PD-L1-targeted therapies.
Keywords: biomarker; cancer immunotherapy; companion diagnostic; immune checkpoint inhibitor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Liu X, Guo C-Y, Tou F-F, Wen X-M, Kuang Y-K, Zhu Q, Hu H. Association of PD-L1 expression status with the efficacy of PD-1/PD-L1 inhibitors and overall survival in solid tumours: a systematic review and meta-analysis. International Journal of Cancer. 2020;147(1):116–127. - PubMed
-
- FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and other Tools) Resource [updated 11/13/2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK338448/. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

