SDF-1α gene-activated collagen scaffold enhances provasculogenic response in a coculture of human endothelial cells with human adipose-derived stromal cells
- PMID: 33677751
- PMCID: PMC7936958
- DOI: 10.1007/s10856-021-06499-6
SDF-1α gene-activated collagen scaffold enhances provasculogenic response in a coculture of human endothelial cells with human adipose-derived stromal cells
Abstract
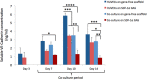
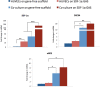
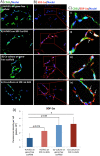
Novel biomaterials can be used to provide a better environment for cross talk between vessel forming endothelial cells and wound healing instructor stem cells for tissue regeneration. This study seeks to investigate if a collagen scaffold containing a proangiogenic gene encoding for the chemokine stromal-derived factor-1 alpha (SDF-1α GAS) could be used to enhance functional responses in a coculture of human umbilical vein endothelial cells (HUVECs) and human adipose-derived stem/stromal cells (ADSCs). Functional responses were determined by (1) monitoring the amount of junctional adhesion molecule VE-cadherin released during 14 days culture, (2) expression of provasculogenic genes on the 14th day, and (3) the bioactivity of secreted factors on neurogenic human Schwann cells. When we compared our SDF-1α GAS with a gene-free scaffold, the results showed positive proangiogenic determination characterized by a transient yet controlled release of the VE-cadherin. On the 14th day, the coculture on the SDF-1α GAS showed enhanced maturation than its gene-free equivalent through the elevation of provasculogenic genes (SDF-1α-7.4-fold, CXCR4-1.5-fold, eNOS-1.5-fold). Furthermore, we also found that the coculture on SDF-1α GAS secretes bioactive factors that significantly (p < 0.01) enhanced human Schwann cells' clustering to develop toward Bünger band-like structures. Conclusively, this study reports that SDF-1α GAS could be used to produce a bioactive vascularized construct through the enhancement of the cooperative effects between endothelial cells and ADSCs.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
SDF-1α gene-activated collagen scaffold drives functional differentiation of human Schwann cells for wound healing applications.Biotechnol Bioeng. 2021 Feb;118(2):725-736. doi: 10.1002/bit.27601. Epub 2020 Nov 9. Biotechnol Bioeng. 2021. PMID: 33064302
-
Pro-angiogenic impact of SDF-1α gene-activated collagen-based scaffolds in stem cell driven angiogenesis.Int J Pharm. 2018 Jun 15;544(2):372-379. doi: 10.1016/j.ijpharm.2018.03.032. Epub 2018 Mar 17. Int J Pharm. 2018. PMID: 29555441
-
Sustained release of collagen-affinity SDF-1α from book-shaped acellular fibrocartilage scaffold enhanced bone-tendon healing in a rabbit model.J Orthop Res. 2021 Jun;39(6):1331-1343. doi: 10.1002/jor.24687. Epub 2020 Apr 17. J Orthop Res. 2021. PMID: 32275087
-
Mineralised Collagen Scaffolds Loaded with Stromal Cell-derived Factor-1 Improve Mandibular Bone Regeneration.Chin J Dent Res. 2014;17(1):23-9. Chin J Dent Res. 2014. PMID: 25028686
-
The effect of incorporation of exogenous stromal cell-derived factor-1 alpha within a knitted silk-collagen sponge scaffold on tendon regeneration.Biomaterials. 2010 Oct;31(28):7239-49. doi: 10.1016/j.biomaterials.2010.05.040. Biomaterials. 2010. PMID: 20615544
Cited by
-
Immunomodulatory biomaterials on chemokine signaling in wound healing.Front Pharmacol. 2023 Apr 21;14:1084948. doi: 10.3389/fphar.2023.1084948. eCollection 2023. Front Pharmacol. 2023. PMID: 37153787 Free PMC article. Review.
-
Dual delivery gene-activated scaffold directs fibroblast activity and keratinocyte epithelization.APL Bioeng. 2024 Jan 26;8(1):016104. doi: 10.1063/5.0174122. eCollection 2024 Mar. APL Bioeng. 2024. PMID: 38283135 Free PMC article.
-
Anti-Aging β-Klotho Gene-Activated Scaffold Promotes Rejuvenative Wound Healing Response in Human Adipose-Derived Stem Cells.Pharmaceuticals (Basel). 2021 Nov 17;14(11):1168. doi: 10.3390/ph14111168. Pharmaceuticals (Basel). 2021. PMID: 34832950 Free PMC article.
References
-
- Quinlan E, López‐Noriega A, Thompson EM, Hibbitts A, Cryan SA, O’Brien FJ. Controlled release of vascular endothelial growth factor from spray‐dried alginate microparticles in collagen–hydroxyapatite scaffolds for promoting vascularization and bone repair. J Tissue Eng Regen Med. 2017;11:1097–109. doi: 10.1002/term.2013. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

