COVID-19 vaccines: rapid development, implications, challenges and future prospects
- PMID: 33677814
- PMCID: PMC7937046
- DOI: 10.1007/s13577-021-00512-4
COVID-19 vaccines: rapid development, implications, challenges and future prospects
Abstract
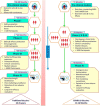
COVID-19 has affected millions of people and put an unparalleled burden on healthcare systems as well as economies throughout the world. Currently, there is no decisive therapy for COVID-19 or related complications. The only hope to mitigate this pandemic is through vaccines. The COVID-19 vaccines are being developed rapidly, compared to traditional vaccines, and are being approved via Emergency Use Authorization (EUA) worldwide. So far, there are 232 vaccine candidates. One hundred and seventy-two are in preclinical development and 60 in clinical development, of which 9 are approved under EUA by different countries. This includes the United Kingdom (UK), United States of America (USA), Canada, Russia, China, and India. Distributing vaccination to all, with a safe and efficacious vaccine is the leading priority for all nations to combat this COVID-19 pandemic. However, the current accelerated process of COVID-19 vaccine development and EUA has many unanswered questions. In addition, the change in strain of SARS-CoV-2 in UK and South Africa, and its increasing spread across the world have raised more challenges, both for the vaccine developers as well as the governments across the world. In this review, we have discussed the different type of vaccines with examples of COVID-19 vaccines, their rapid development compared to the traditional vaccine, associated challenges, and future prospects.
Keywords: COVID-19; COVID-19 vaccine; Emergency use authorization; SARS-Cov-2; Vaccine hesitancy.
Conflict of interest statement
The authors declare that they no conflict of interest.
Figures
References
-
- WHO Coronavirus Disease (COVID-19) Dashboard [Internet]. WHO (World Heal. Organ. 2021. Available from: https://covid19.who.int/. Cited 3 Jan 2021.
-
- Draft Landscape of COVID-19 candidate vaccines—29 December 2020 [Internet]. World Heal. Organ. 2020, p. 12. Available from: www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-va.... Cited 30 Dec 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous