Ultrafast Directional Janus Pt-Mesoporous Silica Nanomotors for Smart Drug Delivery
- PMID: 33677957
- PMCID: PMC8719758
- DOI: 10.1021/acsnano.0c08404
Ultrafast Directional Janus Pt-Mesoporous Silica Nanomotors for Smart Drug Delivery
Abstract
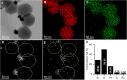
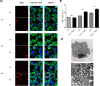
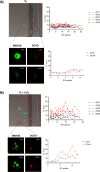
Development of bioinspired nanomachines with an efficient propulsion and cargo-towing has attracted much attention in the last years due to their potential biosensing, diagnostics, and therapeutics applications. In this context, self-propelled synthetic nanomotors are promising carriers for intelligent and controlled release of therapeutic payloads. However, the implementation of this technology in real biomedical applications is still facing several challenges. Herein, we report the design, synthesis, and characterization of innovative multifunctional gated platinum-mesoporous silica nanomotors constituted of a propelling element (platinum nanodendrite face), a drug-loaded nanocontainer (mesoporous silica nanoparticle face), and a disulfide-containing oligo(ethylene glycol) chain (S-S-PEG) as a gating system. These Janus-type nanomotors present an ultrafast self-propelled motion due to the catalytic decomposition of low concentrations of hydrogen peroxide. Likewise, nanomotors exhibit a directional movement, which drives the engines toward biological targets, THP-1 cancer cells, as demonstrated using a microchip device that mimics penetration from capillary to postcapillary vessels. This fast and directional displacement facilitates the rapid cellular internalization and the on-demand specific release of a cytotoxic drug into the cytosol, due to the reduction of the disulfide bonds of the capping ensemble by intracellular glutathione levels. In the microchip device and in the absence of fuel, nanomotors are neither able to move directionally nor reach cancer cells and deliver their cargo, revealing that the fuel is required to get into inaccessible areas and to enhance nanoparticle internalization and drug release. Our proposed nanosystem shows many of the suitable characteristics for ideal biomedical destined nanomotors, such as rapid autonomous motion, versatility, and stimuli-responsive controlled drug release.
Keywords: Janus nanomotors; directional motion; drug delivery; on-command controlled release; ultrafast self-propulsion.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Ficin-Cyclodextrin-Based Docking Nanoarchitectonics of Self-Propelled Nanomotors for Bacterial Biofilm Eradication.Chem Mater. 2023 May 9;35(11):4412-4426. doi: 10.1021/acs.chemmater.3c00587. eCollection 2023 Jun 13. Chem Mater. 2023. PMID: 37332683 Free PMC article.
-
Glucose-Fueled Gated Nanomotors: Enhancing In Vivo Anticancer Efficacy via Deep Drug Penetration into Tumors.ACS Nano. 2025 Jun 10;19(22):20932-20955. doi: 10.1021/acsnano.5c03799. Epub 2025 May 30. ACS Nano. 2025. PMID: 40444744 Free PMC article.
-
Multi-phoretic nanomotor with consistent motion direction for enhanced cancer therapy.Acta Biomater. 2025 Jan 1;191:352-368. doi: 10.1016/j.actbio.2024.11.037. Epub 2024 Nov 24. Acta Biomater. 2025. PMID: 39586348
-
Unfolding the future: Self-controlled catalytic nanomotor in healthcare system.Mater Sci Eng C Mater Biol Appl. 2020 Dec;117:111330. doi: 10.1016/j.msec.2020.111330. Epub 2020 Aug 7. Mater Sci Eng C Mater Biol Appl. 2020. PMID: 32919683 Review.
-
Catalytically propelled micro-/nanomotors: how fast can they move?Chem Rec. 2012 Feb;12(1):224-31. doi: 10.1002/tcr.201100031. Epub 2011 Dec 9. Chem Rec. 2012. PMID: 22162283 Review.
Cited by
-
Multifunctional mesoporous silica nanoparticles for biomedical applications.Signal Transduct Target Ther. 2023 Nov 24;8(1):435. doi: 10.1038/s41392-023-01654-7. Signal Transduct Target Ther. 2023. PMID: 37996406 Free PMC article. Review.
-
Biological Use of Nanostructured Silica-Based Materials Functionalized with Metallodrugs: The Spanish Perspective.Int J Mol Sci. 2023 Jan 25;24(3):2332. doi: 10.3390/ijms24032332. Int J Mol Sci. 2023. PMID: 36768659 Free PMC article. Review.
-
Plasmon enhanced catalysis-driven nanomotors with autonomous navigation for deep cancer imaging and enhanced radiotherapy.Chem Sci. 2022 Oct 12;13(43):12840-12850. doi: 10.1039/d2sc03036e. eCollection 2022 Nov 9. Chem Sci. 2022. PMID: 36519050 Free PMC article.
-
Review of Bubble Applications in Microrobotics: Propulsion, Manipulation, and Assembly.Micromachines (Basel). 2022 Jul 4;13(7):1068. doi: 10.3390/mi13071068. Micromachines (Basel). 2022. PMID: 35888885 Free PMC article. Review.
-
Diffusiophoresis of a Weakly Charged Liquid Metal Droplet.Molecules. 2023 May 5;28(9):3905. doi: 10.3390/molecules28093905. Molecules. 2023. PMID: 37175315 Free PMC article.
References
-
- Pijpers I. A. B.; Cao S.; Llopis-Lorente A.; Zhu J.; Song S.; Joosten R. R. M.; Meng F.; Friedrich H.; Williams D. S.; Sanchez S.; van Hest J. C. M.; Abdelmohsen L. K. E. A. Hybrid Biodegradable Nanomotors through Compartmentalized Synthesis. Nano Lett. 2020, 20, 4472–4480. 10.1021/acs.nanolett.0c01268. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

