No difference in effects of 'PACE steps to success' palliative care program for nursing home residents with and without dementia: a pre-planned subgroup analysis of the seven-country PACE trial
- PMID: 33678179
- PMCID: PMC7937240
- DOI: 10.1186/s12904-021-00734-1
No difference in effects of 'PACE steps to success' palliative care program for nursing home residents with and without dementia: a pre-planned subgroup analysis of the seven-country PACE trial
Abstract
Background: 'PACE Steps to Success' is a multicomponent training program aiming to integrate generalist and non-disease-specific palliative care in nursing homes. This program did not improve residents' comfort in the last week of life, but it appeared to improve quality of care and dying in their last month of life. Because this program included only three dementia-specific elements, its effects might differ depending on the presence or stage of dementia. We aimed to investigate whether the program effects differ between residents with advanced, non-advanced, and no dementia.
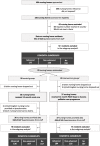
Methods: Pre-planned subgroup analysis of the PACE cluster-randomized controlled trial in 78 nursing homes in seven European countries. Participants included residents who died in the previous 4 months. The nursing home staff or general practitioner assessed the presence of dementia; severity was determined using two highly-discriminatory staff-reported instruments. Using after-death questionnaires, staff assessed comfort in the last week of life (Comfort Assessment in Dying-End-of-Life in Dementia-scale; primary outcome) and quality of care and dying in the last month of life (Quality of Dying in Long-Term Care scale; secondary outcome).
Results: At baseline, we included 177 residents with advanced dementia, 126 with non-advanced dementia and 156 without dementia. Post-intervention, respectively in the control and the intervention group, we included 136 and 104 residents with advanced dementia, 167 and 110 with non-advanced dementia and 157 and 137 without dementia. We found no subgroup differences on comfort in the last week of life, comparing advanced versus without dementia (baseline-adjusted mean sub-group difference 2.1; p-value = 0.177), non-advanced versus without dementia (2.7; p = 0.092), and advanced versus non-advanced dementia (- 0.6; p = 0.698); or on quality of care and dying in the last month of life, comparing advanced and without dementia (- 0.6; p = 0.741), non-advanced and without dementia (- 1.5; p = 0.428), and advanced and non-advanced dementia (0.9; p = 0.632).
Conclusions: The lack of subgroup difference suggests that while the program did not improve comfort in dying residents with or without dementia, it appeared to equally improve quality of care and dying in the last month of life for residents with dementia (regardless of the stage) and those without dementia. A generalist and non-disease-specific palliative care program, such as PACE Steps to Success, is a useful starting point for future palliative care improvement in nursing homes, but to effectively improve residents' comfort, this program needs further development.
Trial registration: ISRCTN, ISRCTN14741671 . Registered 8 July 2015 - Retrospectively registered.
Keywords: Bereavement; Communication; End of life care; Neurological conditions; Nursing home care; Pain.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures
References
-
- Broad JB, Gott M, Kim H, et al. Where do people die? An international comparison of the percentage of deaths occurring in hospital and residential aged care settings in 45 populations , using published and available statistics. Int J Public Health. 2013;58:257–267. doi: 10.1007/s00038-012-0394-5. - DOI - PubMed
-
- Froggatt K, Edwards M, Morbey H, Payne S. PACE Work Package 1 and EAPC Taskforce Report. 2016. Mapping palliative care systems in long term care facilities in Europe.
-
- Smets T, Onwuteaka-Philipsen BBD, Miranda R, et al. Integrating palliative care in long-term care facilities across Europe (PACE): protocol of a cluster randomized controlled trial of the “PACE steps to success” intervention in seven countries. BMC Palliat Care. 2018;17(1):1–11. doi: 10.1186/s12904-018-0297-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

