Hyperuricemia in Kidney Disease: A Major Risk Factor for Cardiovascular Events, Vascular Calcification, and Renal Damage
- PMID: 33678312
- PMCID: PMC7951176
- DOI: 10.1016/j.semnephrol.2020.12.004
Hyperuricemia in Kidney Disease: A Major Risk Factor for Cardiovascular Events, Vascular Calcification, and Renal Damage
Abstract
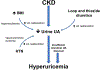
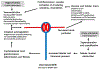
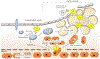
Kidney disease, especially when it is associated with a reduction in estimated glomerular filtration rate, can be associated with an increase in serum urate (uric acid), suggesting that hyperuricemia in subjects with kidney disease may be a strictly secondary phenomenon. Mendelian randomization studies that evaluate genetic scores regulating serum urate also generally have not found evidence that serum urate is a causal risk factor in chronic kidney disease. Nevertheless, this is countered by a large number of epidemiologic, experimental, and clinical studies that have suggested a potentially important role for uric acid in kidney disease and cardiovascular disease. Here, we review the topic in detail. Overall, the studies strongly suggest that hyperuricemia does have an important pathogenic role that likely is driven by intracellular urate levels. An exception may be the role of extracellular uric acid in atherosclerosis and vascular calcification. One of the more striking findings on reviewing the literature is that the primary benefit of lowering serum urate in subjects with CKD is not by slowing the progression of renal disease, but rather by reducing the incidence of cardiovascular events and mortality. We recommend large-scale clinical trials to determine if there is a benefit in lowering serum urate in hyperuricemic subjects in acute and chronic kidney disease and in the reduction of cardiovascular morbidity and mortality in subjects with end-stage chronic kidney disease.
Keywords: Hyperuricemia; acute kidney injury; allopurinol; cardiovascular mortality; chronic kidney disease.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
References
-
- Johnson G: On the Diseases of the Kidney. London, John W Parker and Son, 1852.
-
- Haig A: Uric acid as a Factor in the Causation of Disease. A contribution to the pathology of high arterial tension, headache, epilepsy, mental depression, gout, rheumatism, diabetes, Bright’s disease, and other disorders., ed First. London, J & A Churchill, 1892.
-
- Davis NC: The cardiovascular and renal relations and manifestations of gout. JAMA 1897;29:261–262.
-
- Fishberg AM: The interpretation of increased blood uric acid in hypertension. Arch Intern Med 1924;34:503–507.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

