Glypicans and Heparan Sulfate in Synaptic Development, Neural Plasticity, and Neurological Disorders
- PMID: 33679334
- PMCID: PMC7928303
- DOI: 10.3389/fncir.2021.595596
Glypicans and Heparan Sulfate in Synaptic Development, Neural Plasticity, and Neurological Disorders
Abstract
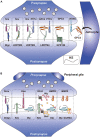
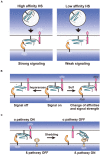
Heparan sulfate proteoglycans (HSPGs) are components of the cell surface and extracellular matrix, which bear long polysaccharides called heparan sulfate (HS) attached to the core proteins. HSPGs interact with a variety of ligand proteins through the HS chains, and mutations in HSPG-related genes influence many biological processes and cause various diseases. In particular, recent findings from vertebrate and invertebrate studies have raised the importance of glycosylphosphatidylinositol-anchored HSPGs, glypicans, as central players in the development and functions of synapses. Glypicans are important components of the synapse-organizing protein complexes and serve as ligands for leucine-rich repeat transmembrane neuronal proteins (LRRTMs), leukocyte common antigen-related (LAR) family receptor protein tyrosine phosphatases (RPTPs), and G-protein-coupled receptor 158 (GPR158), regulating synapse formation. Many of these interactions are mediated by the HS chains of glypicans. Neurexins (Nrxs) are also synthesized as HSPGs and bind to some ligands in common with glypicans through HS chains. Therefore, glypicans and Nrxs may act competitively at the synapses. Furthermore, glypicans regulate the postsynaptic expression levels of ionotropic glutamate receptors, controlling the electrophysiological properties and non-canonical BMP signaling of synapses. Dysfunctions of glypicans lead to failures in neuronal network formation, malfunction of synapses, and abnormal behaviors that are characteristic of neurodevelopmental disorders. Recent human genetics revealed that glypicans and HS are associated with autism spectrum disorder, neuroticism, and schizophrenia. In this review, we introduce the studies showing the roles of glypicans and HS in synapse formation, neural plasticity, and neurological disorders, especially focusing on the mouse and Drosophila as potential models for human diseases.
Keywords: autism spectrum disorder; glypican; heparan sulfate proteoglycan; neurexin; schizophrenia; synapse-organizing protein; synaptic plasticity.
Copyright © 2021 Kamimura and Maeda.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Heparan sulfate proteoglycans in Drosophila neuromuscular development.Biochim Biophys Acta Gen Subj. 2017 Oct;1861(10):2442-2446. doi: 10.1016/j.bbagen.2017.06.015. Epub 2017 Jun 20. Biochim Biophys Acta Gen Subj. 2017. PMID: 28645846 Review.
-
Role of LRRTMs in synapse development and plasticity.Neurosci Res. 2017 Mar;116:18-28. doi: 10.1016/j.neures.2016.10.003. Epub 2016 Oct 31. Neurosci Res. 2017. PMID: 27810425 Review.
-
Combinatorial roles of heparan sulfate proteoglycans and heparan sulfates in Caenorhabditis elegans neural development.PLoS One. 2014 Jul 23;9(7):e102919. doi: 10.1371/journal.pone.0102919. eCollection 2014. PLoS One. 2014. PMID: 25054285 Free PMC article.
-
Differential heparan sulfate dependency of the Drosophila glypicans.J Biol Chem. 2024 Jan;300(1):105544. doi: 10.1016/j.jbc.2023.105544. Epub 2023 Dec 10. J Biol Chem. 2024. PMID: 38072044 Free PMC article.
-
The Glycosaminoglycan Side Chains and Modular Core Proteins of Heparan Sulphate Proteoglycans and the Varied Ways They Provide Tissue Protection by Regulating Physiological Processes and Cellular Behaviour.Int J Mol Sci. 2023 Sep 14;24(18):14101. doi: 10.3390/ijms241814101. Int J Mol Sci. 2023. PMID: 37762403 Free PMC article. Review.
Cited by
-
Biallelic variants in the SLC13A1 sulfate transporter gene cause hyposulfatemia with a mild spondylo-epi-metaphyseal dysplasia.Clin Genet. 2023 Jan;103(1):45-52. doi: 10.1111/cge.14239. Epub 2022 Oct 3. Clin Genet. 2023. PMID: 36175384 Free PMC article.
-
Post-synaptic specialization of the neuromuscular junction: junctional folds formation, function, and disorders.Cell Biosci. 2022 Jun 19;12(1):93. doi: 10.1186/s13578-022-00829-z. Cell Biosci. 2022. PMID: 35718785 Free PMC article. Review.
-
HS, an Ancient Molecular Recognition and Information Storage Glycosaminoglycan, Equips HS-Proteoglycans with Diverse Matrix and Cell-Interactive Properties Operative in Tissue Development and Tissue Function in Health and Disease.Int J Mol Sci. 2023 Jan 6;24(2):1148. doi: 10.3390/ijms24021148. Int J Mol Sci. 2023. PMID: 36674659 Free PMC article. Review.
-
Circadian rhythm disruptions associated with opioid use disorder in synaptic proteomes of human dorsolateral prefrontal cortex and nucleus accumbens.Mol Psychiatry. 2023 Nov;28(11):4777-4792. doi: 10.1038/s41380-023-02241-6. Epub 2023 Sep 6. Mol Psychiatry. 2023. PMID: 37674018 Free PMC article.
-
Neuromodulation technologies improve functional recovery after brain injury: From bench to bedside.Neural Regen Res. 2026 Feb 1;21(2):506-520. doi: 10.4103/NRR.NRR-D-24-00652. Epub 2024 Dec 7. Neural Regen Res. 2026. PMID: 39851132 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

