Distinct Spiking Patterns of Excitatory and Inhibitory Neurons and LFP Oscillations in Prefrontal Cortex During Sensory Discrimination
- PMID: 33679434
- PMCID: PMC7928411
- DOI: 10.3389/fphys.2021.618307
Distinct Spiking Patterns of Excitatory and Inhibitory Neurons and LFP Oscillations in Prefrontal Cortex During Sensory Discrimination
Abstract
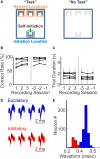
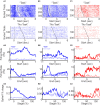
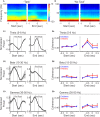
Prefrontal cortex (PFC) are broadly linked to various aspects of behavior. During sensory discrimination, PFC neurons can encode a range of task related information, including the identity of sensory stimuli and related behavioral outcome. However, it remains largely unclear how different neuron subtypes and local field potential (LFP) oscillation features in the mouse PFC are modulated during sensory discrimination. To understand how excitatory and inhibitory PFC neurons are selectively engaged during sensory discrimination and how their activity relates to LFP oscillations, we used tetrode recordings to probe well-isolated individual neurons, and LFP oscillations, in mice performing a three-choice auditory discrimination task. We found that a majority of PFC neurons, 78% of the 711 recorded individual neurons, exhibited sensory discrimination related responses that are context and task dependent. Using spike waveforms, we classified these responsive neurons into putative excitatory neurons with broad waveforms or putative inhibitory neurons with narrow waveforms, and found that both neuron subtypes were transiently modulated, with individual neurons' responses peaking throughout the entire duration of the trial. While the number of responsive excitatory neurons remain largely constant throughout the trial, an increasing fraction of inhibitory neurons were gradually recruited as the trial progressed. Further examination of the coherence between individual neurons and LFPs revealed that inhibitory neurons exhibit higher spike-field coherence with LFP oscillations than excitatory neurons during all aspects of the trial and across multiple frequency bands. Together, our results demonstrate that PFC excitatory neurons are continuously engaged during sensory discrimination, whereas PFC inhibitory neurons are increasingly recruited as the trial progresses and preferentially coordinated with LFP oscillations. These results demonstrate increasing involvement of inhibitory neurons in shaping the overall PFC dynamics toward the completion of the sensory discrimination task.
Keywords: LFP oscillations; auditory discrimination; rodent prefrontal cortex; single unit activity; spike field coherence.
Copyright © 2021 Tseng and Han.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

