Multiscale and Multimodal Optical Imaging of the Ultrastructure of Human Liver Biopsies
- PMID: 33679449
- PMCID: PMC7925637
- DOI: 10.3389/fphys.2021.637136
Multiscale and Multimodal Optical Imaging of the Ultrastructure of Human Liver Biopsies
Abstract
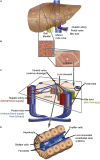
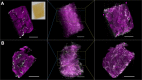
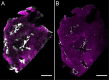
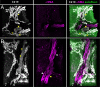
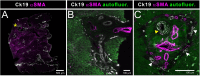
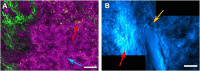
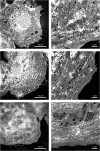
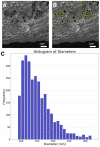
The liver as the largest organ in the human body is composed of a complex macroscopic and microscopic architecture that supports its indispensable function to maintain physiological homeostasis. Optical imaging of the human liver is particularly challenging because of the need to cover length scales across 7 orders of magnitude (from the centimeter scale to the nanometer scale) in order to fully assess the ultrastructure of the entire organ down to the subcellular scale and probe its physiological function. This task becomes even more challenging the deeper within the organ one hopes to image, because of the strong absorption and scattering of visible light by the liver. Here, we demonstrate how optical imaging methods utilizing highly specific fluorescent labels, as well as label-free optical methods can seamlessly cover this entire size range in excised, fixed human liver tissue and we exemplify this by reconstructing the biliary tree in three-dimensional space. Imaging of tissue beyond approximately 0.5 mm length requires optical clearing of the human liver. We present the successful use of optical projection tomography and light-sheet fluorescence microscopy to derive information about the liver architecture on the millimeter scale. The intermediate size range is covered using label-free structural and chemically sensitive methods, such as second harmonic generation and coherent anti-Stokes Raman scattering microscopy. Laser-scanning confocal microscopy extends the resolution to the nanoscale, allowing us to ultimately image individual liver sinusoidal endothelial cells and their fenestrations by super-resolution structured illumination microscopy. This allowed us to visualize the human hepatobiliary system in 3D down to the cellular level, which indicates that reticular biliary networks communicate with portal bile ducts via single or a few ductuli. Non-linear optical microscopy enabled us to identify fibrotic regions extending from the portal field to the parenchyma, along with microvesicular steatosis in liver biopsies from an older patient. Lastly, super-resolution microscopy allowed us to visualize and determine the size distribution of fenestrations in human liver sinusoidal endothelial cells for the first time under aqueous conditions. Thus, this proof-of-concept study allows us to demonstrate, how, in combination, these techniques open up a new chapter in liver biopsy analysis.
Keywords: coherent Raman scattering microscopy; light-sheet fluorescence microscopy; liver biology; liver morphology; liver sinusoidal endothelial cells.; liver sinusoids; super-resolution optical microscopy.
Copyright © 2021 Kong, Bobe, Pilger, Lachetta, Øie, Kirschnick, Mönkemöller, Hübner, Förster, Schüttpelz, Kiefer, Huser and Schulte am Esch.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Multimodal super-resolution optical microscopy visualizes the close connection between membrane and the cytoskeleton in liver sinusoidal endothelial cell fenestrations.Sci Rep. 2015 Nov 9;5:16279. doi: 10.1038/srep16279. Sci Rep. 2015. PMID: 26549018 Free PMC article.
-
Implementation of a Coherent Anti-Stokes Raman Scattering (CARS) System on a Ti:Sapphire and OPO Laser Based Standard Laser Scanning Microscope.J Vis Exp. 2016 Jul 17;(113). doi: 10.3791/54262. J Vis Exp. 2016. PMID: 27501285
-
3D imaging of cleared human skin biopsies using light-sheet microscopy: A new way to visualize in-depth skin structure.Skin Res Technol. 2018 May;24(2):294-303. doi: 10.1111/srt.12429. Epub 2018 Jan 27. Skin Res Technol. 2018. PMID: 29377352
-
Cooperation of liver cells in health and disease.Adv Anat Embryol Cell Biol. 2001;161:III-XIII, 1-151. doi: 10.1007/978-3-642-56553-3. Adv Anat Embryol Cell Biol. 2001. PMID: 11729749 Review.
-
Can Developments in Tissue Optical Clearing Aid Super-Resolution Microscopy Imaging?Int J Mol Sci. 2021 Jun 23;22(13):6730. doi: 10.3390/ijms22136730. Int J Mol Sci. 2021. PMID: 34201632 Free PMC article. Review.
Cited by
-
From Seeing to Simulating: A Survey of Imaging Techniques and Spatially-Resolved Data for Developing Multiscale Computational Models of Liver Regeneration.Front Syst Biol. 2022;2:917191. doi: 10.3389/fsysb.2022.917191. Epub 2022 Jun 6. Front Syst Biol. 2022. PMID: 37575468 Free PMC article.
-
The wHole Story About Fenestrations in LSEC.Front Physiol. 2021 Sep 13;12:735573. doi: 10.3389/fphys.2021.735573. eCollection 2021. Front Physiol. 2021. PMID: 34588998 Free PMC article. Review.
-
Visualization and functional characterization of lymphoid organ fibroblasts.Immunol Rev. 2022 Mar;306(1):108-122. doi: 10.1111/imr.13051. Epub 2021 Dec 5. Immunol Rev. 2022. PMID: 34866192 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

