Skeletal Muscle in Cerebral Palsy: From Belly to Myofibril
- PMID: 33679586
- PMCID: PMC7930059
- DOI: 10.3389/fneur.2021.620852
Skeletal Muscle in Cerebral Palsy: From Belly to Myofibril
Abstract
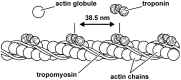
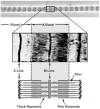
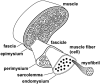
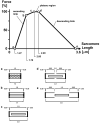
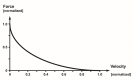
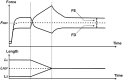
This review will provide a comprehensive, up-to-date review of the current knowledge regarding the pathophysiology of muscle contractures in cerebral palsy. Although much has been known about the clinical manifestations of both dynamic and static muscle contractures, until recently, little was known about the underlying mechanisms for the development of such contractures. In particular, recent basic science and imaging studies have reported an upregulation of collagen content associated with muscle stiffness. Paradoxically, contractile elements such as myofibrils have been found to be highly elastic, possibly an adaptation to a muscle that is under significant in vivo tension. Sarcomeres have also been reported to be excessively long, likely responsible for the poor force generating capacity and underlying weakness seen in children with cerebral palsy (CP). Overall muscle volume and length have been found to be decreased in CP, likely secondary to abnormalities in sarcomerogenesis. Recent animal and clinical work has suggested that the use of botulinum toxin for spasticity management has been shown to increase muscle atrophy and fibrofatty content in the CP muscle. Given that the CP muscle is short and small already, this calls into question the use of such agents for spasticity management given the functional and histological cost of such interventions. Recent theories involving muscle homeostasis, epigenetic mechanisms, and inflammatory mediators of regulation have added to our emerging understanding of this complicated area.
Keywords: cerebral palsy; connective tissue; epigenetics; muscle contractures; muscle morphology; satellite cells; skeletal muscle; spasticity.
Copyright © 2021 Howard and Herzog.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Lance JW. Symposium. In: Feldman RG, Young RR, Koella P. editors. Spasticity: Disordered Motor Control. Chicago, IL: Year Book Medical Pubs; (1980). p. 485-494.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

