Conditional Fgfr1 Deletion in GnRH Neurons Leads to Minor Disruptions in the Reproductive Axis of Male and Female Mice
- PMID: 33679600
- PMCID: PMC7933197
- DOI: 10.3389/fendo.2020.588459
Conditional Fgfr1 Deletion in GnRH Neurons Leads to Minor Disruptions in the Reproductive Axis of Male and Female Mice
Abstract
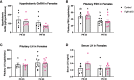
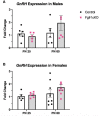
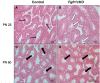
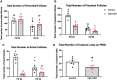
In humans and mice, inactivating mutations in fibroblast growth factor receptor 1 (Fgfr1) lead to gonadotropin-releasing hormone (GnRH) deficiency and a host of downstream reproductive disorders. It was unclear if Fgfr1 signaling directly upon GnRH neurons critically drove the establishment of a functional GnRH system. To answer this question, we generated a mouse model with a conditional deletion of Fgfr1 in GnRH neurons using the Cre/loxP approach. These mice, called Fgfr1cKO mice, were examined along with control mice for their pubertal onset and a host of reproductive axis functions. Our results showed that Fgfr1cKO mice harbored no detectable defects in the GnRH system and pubertal onset, suffered only subtle changes in the pituitary function, but exhibited significantly disrupted testicular and ovarian morphology at 25 days of age, indicating impaired gametogenesis at a young age. However, these disruptions were transient and became undetectable in older mice. Our results suggest that Fgfr1 signaling directly on GnRH neurons supports, to some extent, the reproductive axis function in the period leading to the early phase of puberty, but is not critically required for pubertal onset or reproductive maintenance in sexually mature animals.
Keywords: Kallmann syndrome; conditional deletion; congenital hypogonadotropic hypogonadism; fibroblast growth factor receptor 1; gonadotropin-releasing hormone neurons; hypothalamic-pituitary gonadal axis.
Copyright © 2021 Dela Cruz, Horton, Sanders, Andersen and Tsai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Compound deficiencies in multiple fibroblast growth factor signalling components differentially impact the murine gonadotrophin-releasing hormone system.J Neuroendocrinol. 2010 Aug;22(8):944-50. doi: 10.1111/j.1365-2826.2010.02024.x. Epub 2010 Jun 9. J Neuroendocrinol. 2010. PMID: 20553372 Free PMC article.
-
Fibroblast growth factor 8 signaling through fibroblast growth factor receptor 1 is required for the emergence of gonadotropin-releasing hormone neurons.Endocrinology. 2008 Oct;149(10):4997-5003. doi: 10.1210/en.2007-1634. Epub 2008 Jun 19. Endocrinology. 2008. PMID: 18566132 Free PMC article.
-
KLB, encoding β-Klotho, is mutated in patients with congenital hypogonadotropic hypogonadism.EMBO Mol Med. 2017 Oct;9(10):1379-1397. doi: 10.15252/emmm.201607376. EMBO Mol Med. 2017. PMID: 28754744 Free PMC article.
-
Genetics defects in GNRH1: a paradigm of hypothalamic congenital gonadotropin deficiency.Brain Res. 2010 Dec 10;1364:3-9. doi: 10.1016/j.brainres.2010.09.084. Epub 2010 Sep 29. Brain Res. 2010. PMID: 20887715 Review.
-
[GnRH deficiency: new insights from genetics].J Soc Biol. 2004;198(1):80-7. J Soc Biol. 2004. PMID: 15146960 Review. French.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

