A Sensitive Plasma Insulin Immunoassay to Establish the Diagnosis of Congenital Hyperinsulinism
- PMID: 33679602
- PMCID: PMC7935514
- DOI: 10.3389/fendo.2020.614993
A Sensitive Plasma Insulin Immunoassay to Establish the Diagnosis of Congenital Hyperinsulinism
Abstract
Background: The diagnosis of congenital hyperinsulinism (CHI) may be hampered by a plasma (p-) insulin detection limit of 12-18 pmol/L (2-3 mU/L).
Objective: To evaluate the diagnostic performance of a sensitive insulin immunoassay and to find the optimal p-insulin cut-off for the diagnosis of CHI.
Methods: Diagnostic fasting tests, performed without medication or i.v.-glucose, were investigated in children with a clinical diagnosis of CHI, or idiopathic ketotic hypoglycemia (IKH). The CHI diagnosis was either clinical or by the alternative, p-insulin-free criteria; hypoglycemia plus disease-causing genetic mutations and/or CHI-compatible pancreatic histopathology. We included diagnostic p-insulin samples with simultaneous p-glucose <3.2 mmol/L and used a sensitive insulin assay (Cobas e411 immunoassay analyzer; lower detection limit 1.2 pmol/L; normal range 15.1-147.1 pmol/L). Receiver operating characteristics area under the curve (ROC AUC) values and optimal cut-offs were analyzed for the performance of p-insulin to diagnose CHI.
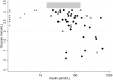
Results: In 61 CHI patients, the median (range) p-insulin was 76.5 (17-644) pmol/L compared to 1.5 (1.5-7.7) pmol/L in IKH patients (n=15). The ROC AUC was 1.0 for the diagnosis of CHI defined both by the clinical diagnosis (n=61) and by alternative criteria (n=57). The optimal p-insulin cut-offs were 12.3 pmol/L, and 10.6 pmol/L, at p-glucose <3.2 mmol/L (n=61), and <3.0 mmol/L (n=49), respectively.
Conclusions: The sensitive insulin assay performed excellent in diagnosing CHI with optimal p-insulin cut-offs at 12.3 pmol/L (2.0 mU/L), and 10.6 pmol/L (1.8 mU/L), at p-glucose <3.2 mmol/L, and <3.0 mmol/L, respectively. A sensitive insulin assay may serve to simplify the diagnosis of CHI.
Keywords: children; congenital hyperinsulinism; diagnostic performance; hypoglycemia; immunoassays.
Copyright © 2021 Siersbæk, Larsen, Nybo and Christesen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

