Redox Epiphospholipidome in Programmed Cell Death Signaling: Catalytic Mechanisms and Regulation
- PMID: 33679610
- PMCID: PMC7933662
- DOI: 10.3389/fendo.2020.628079
Redox Epiphospholipidome in Programmed Cell Death Signaling: Catalytic Mechanisms and Regulation
Abstract
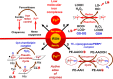
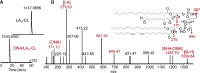
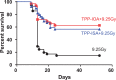
A huge diversification of phospholipids, forming the aqueous interfaces of all biomembranes, cannot be accommodated within a simple concept of their role as membrane building blocks. Indeed, a number of signaling functions of (phospho)lipid molecules has been discovered. Among these signaling lipids, a particular group of oxygenated polyunsaturated fatty acids (PUFA), so called lipid mediators, has been thoroughly investigated over several decades. This group includes oxygenated octadecanoids, eicosanoids, and docosanoids and includes several hundreds of individual species. Oxygenation of PUFA can occur when they are esterified into major classes of phospholipids. Initially, these events have been associated with non-specific oxidative injury of biomembranes. An alternative concept is that these post-synthetically oxidatively modified phospholipids and their adducts with proteins are a part of a redox epiphospholipidome that represents a rich and versatile language for intra- and inter-cellular communications. The redox epiphospholipidome may include hundreds of thousands of individual molecular species acting as meaningful biological signals. This review describes the signaling role of oxygenated phospholipids in programs of regulated cell death. Although phospholipid peroxidation has been associated with almost all known cell death programs, we chose to discuss enzymatic pathways activated during apoptosis and ferroptosis and leading to peroxidation of two phospholipid classes, cardiolipins (CLs) and phosphatidylethanolamines (PEs). This is based on the available LC-MS identification and quantitative information on the respective peroxidation products of CLs and PEs. We focused on molecular mechanisms through which two proteins, a mitochondrial hemoprotein cytochrome c (cyt c), and non-heme Fe lipoxygenase (LOX), change their catalytic properties to fulfill new functions of generating oxygenated CL and PE species. Given the high selectivity and specificity of CL and PE peroxidation we argue that enzymatic reactions catalyzed by cyt c/CL complexes and 15-lipoxygenase/phosphatidylethanolamine binding protein 1 (15LOX/PEBP1) complexes dominate, at least during the initiation stage of peroxidation, in apoptosis and ferroptosis. We contrast cell-autonomous nature of CLox signaling in apoptosis correlating with its anti-inflammatory functions vs. non-cell-autonomous ferroptotic signaling facilitating pro-inflammatory (necro-inflammatory) responses. Finally, we propose that small molecule mechanism-based regulators of enzymatic phospholipid peroxidation may lead to highly specific anti-apoptotic and anti-ferroptotic therapeutic modalities.
Keywords: apoptosis; cardiolipin; cytochrome c; ferroptosis; lipoxygenase; phospholipid peroxidation; redox lipidomics; regulated cell death.
Copyright © 2021 Kagan, Tyurina, Vlasova, Kapralov, Amoscato, Anthonymuthu, Tyurin, Shrivastava, Cinemre, Lamade, Epperly, Greenberger, Beezhold, Mallampalli, Srivastava, Bayir and Shvedova.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Strikingly High Activity of 15-Lipoxygenase Towards Di-Polyunsaturated Arachidonoyl/Adrenoyl-Phosphatidylethanolamines Generates Peroxidation Signals of Ferroptotic Cell Death.Angew Chem Int Ed Engl. 2024 Feb 26;63(9):e202314710. doi: 10.1002/anie.202314710. Epub 2024 Jan 17. Angew Chem Int Ed Engl. 2024. PMID: 38230815 Free PMC article.
-
NO● Represses the Oxygenation of Arachidonoyl PE by 15LOX/PEBP1: Mechanism and Role in Ferroptosis.Int J Mol Sci. 2021 May 17;22(10):5253. doi: 10.3390/ijms22105253. Int J Mol Sci. 2021. PMID: 34067535 Free PMC article.
-
"Only a Life Lived for Others Is Worth Living": Redox Signaling by Oxygenated Phospholipids in Cell Fate Decisions.Antioxid Redox Signal. 2018 Nov 1;29(13):1333-1358. doi: 10.1089/ars.2017.7124. Epub 2017 Oct 16. Antioxid Redox Signal. 2018. PMID: 28835115 Free PMC article. Review.
-
Redox (phospho)lipidomics of signaling in inflammation and programmed cell death.J Leukoc Biol. 2019 Jul;106(1):57-81. doi: 10.1002/JLB.3MIR0119-004RR. Epub 2019 May 9. J Leukoc Biol. 2019. PMID: 31071242 Free PMC article. Review.
-
"Redox lipidomics technology: Looking for a needle in a haystack".Chem Phys Lipids. 2019 Jul;221:93-107. doi: 10.1016/j.chemphyslip.2019.03.012. Epub 2019 Mar 27. Chem Phys Lipids. 2019. PMID: 30928338 Free PMC article. Review.
Cited by
-
Iron Dysregulation in Mitochondrial Dysfunction and Alzheimer's Disease.Antioxidants (Basel). 2022 Mar 31;11(4):692. doi: 10.3390/antiox11040692. Antioxidants (Basel). 2022. PMID: 35453377 Free PMC article. Review.
-
The critical role of mitochondrial lipid peroxidation in ferroptosis: insights from recent studies.Biophys Rev. 2023 Sep 13;15(5):875-885. doi: 10.1007/s12551-023-01126-w. eCollection 2023 Oct. Biophys Rev. 2023. PMID: 37974984 Free PMC article. Review.
-
Ferroptosis is a new therapeutic target for spinal cord injury.Front Neurosci. 2023 Mar 14;17:1136143. doi: 10.3389/fnins.2023.1136143. eCollection 2023. Front Neurosci. 2023. PMID: 36998732 Free PMC article. Review.
-
Vitamin E/Coenzyme Q-Dependent "Free Radical Reductases": Redox Regulators in Ferroptosis.Antioxid Redox Signal. 2024 Feb;40(4-6):317-328. doi: 10.1089/ars.2022.0154. Epub 2023 Oct 16. Antioxid Redox Signal. 2024. PMID: 37154783 Free PMC article. Review.
-
Compartmentalized mitochondrial ferroptosis converges with optineurin-mediated mitophagy to impact airway epithelial cell phenotypes and asthma outcomes.Nat Commun. 2024 Jul 10;15(1):5818. doi: 10.1038/s41467-024-50222-2. Nat Commun. 2024. PMID: 38987265 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

