Evolutionary Dynamics and Dissemination Pattern of the SARS-CoV-2 Lineage B.1.1.33 During the Early Pandemic Phase in Brazil
- PMID: 33679622
- PMCID: PMC7925893
- DOI: 10.3389/fmicb.2020.615280
Evolutionary Dynamics and Dissemination Pattern of the SARS-CoV-2 Lineage B.1.1.33 During the Early Pandemic Phase in Brazil
Abstract
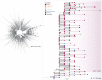
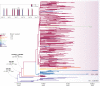
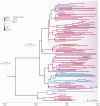
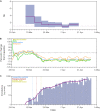
A previous study demonstrates that most of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) Brazilian strains fell in three local clades that were introduced from Europe around late February 2020. Here we investigated in more detail the origin of the major and most widely disseminated SARS-CoV-2 Brazilian lineage B.1.1.33. We recovered 190 whole viral genomes collected from 13 Brazilian states from February 29 to April 31, 2020 and combined them with other B.1.1 genomes collected globally. Our genomic survey confirms that lineage B.1.1.33 is responsible for a variable fraction of the community viral transmissions in Brazilian states, ranging from 2% of all SARS-CoV-2 genomes from Pernambuco to 80% of those from Rio de Janeiro. We detected a moderate prevalence (5-18%) of lineage B.1.1.33 in some South American countries and a very low prevalence (<1%) in North America, Europe, and Oceania. Our study reveals that lineage B.1.1.33 evolved from an ancestral clade, here designated B.1.1.33-like, that carries one of the two B.1.1.33 synapomorphic mutations. The B.1.1.33-like lineage may have been introduced from Europe or arose in Brazil in early February 2020 and a few weeks later gave origin to the lineage B.1.1.33. These SARS-CoV-2 lineages probably circulated during February 2020 and reached all Brazilian regions and multiple countries around the world by mid-March, before the implementation of air travel restrictions in Brazil. Our phylodynamic analysis also indicates that public health interventions were partially effective to control the expansion of lineage B.1.1.33 in Rio de Janeiro because its median effective reproductive number (R e ) was drastically reduced by about 66% during March 2020, but failed to bring it to below one. Continuous genomic surveillance of lineage B.1.1.33 might provide valuable information about epidemic dynamics and the effectiveness of public health interventions in some Brazilian states.
Keywords: Brazil; community transmission; coronavirus; coronavirus disease 2019; genetic lineages; severe acute respiratory syndrome coronavirus-2.
Copyright © 2021 Resende, Delatorre, Gräf, Mir, Motta, Appolinario, Paixão, Mendonça, Ogrzewalska, Caetano, Wallau, Docena, Santos, de Almeida Ferreira, Sousa Junior, Silva, Fernandes, Vianna, Souza, Ferro, Nardy, Santos, Riediger, do Carmo Debur, Croda, Oliveira, Abreu, Bello and Siqueira.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Binny R. N., Lustig A., Brower A., Hendy S. C., James A., Parry M., et al. (2020). Effective reproduction number for COVID-19 in Aotearoa New Zealand. medRxiv [Preprint], 10.1101/2020.08.10.20172320 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

