The Cysteine Protease MaOC1, a Prokaryotic Caspase Homolog, Cleaves the Antitoxin of a Type II Toxin-Antitoxin System
- PMID: 33679669
- PMCID: PMC7935541
- DOI: 10.3389/fmicb.2021.635684
The Cysteine Protease MaOC1, a Prokaryotic Caspase Homolog, Cleaves the Antitoxin of a Type II Toxin-Antitoxin System
Abstract
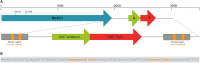
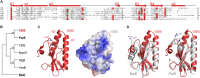
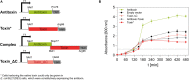
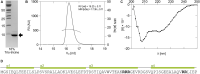
The bloom-forming cyanobacterium Microcystis aeruginosa is known for its global distribution and for the production of toxic compounds. In the genome of M. aeruginosa PCC 7806, we discovered that the gene coding for MaOC1, a caspase homolog protease, is followed by a toxin-antitoxin module, flanked on each side by a direct repeat. We therefore investigated their possible interaction at the protein level. Our results suggest that this module belongs to the ParE/ParD-like superfamily of type II toxin-antitoxin systems. In solution, the antitoxin is predominantly alpha-helical and dimeric. When coexpressed with its cognate toxin and isolated from Escherichia coli, it forms a complex, as revealed by light scattering and affinity purification. The active site of the toxin is restricted to the C-terminus of the molecule. Its truncation led to normal cell growth, while the wild-type form prevented bacterial growth in liquid medium. The orthocaspase MaOC1 was able to cleave the antitoxin so that it could no longer block the toxin activity. The most likely target of the protease was the C-terminus of the antitoxin with two sections of basic amino acid residues. E. coli cells in which MaOC1 was expressed simultaneously with the toxin-antitoxin pair were unable to grow. In contrast, no effect on cell growth was found when using a proteolytically inactive MaOC1 mutant. We thus present the first case of a cysteine protease that regulates the activity of a toxin-antitoxin module, since all currently known activating proteases are of the serine type.
Keywords: Microcystis aeruginosa; ParE/ParD; RelE/ParE; metacaspase; programmed cell death; protease; regulated cell death; toxin-antitoxin system.
Copyright © 2021 Klemenčič, Halužan Vasle and Dolinar.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Orthocaspase and toxin-antitoxin loci rubbing shoulders in the genome of Microcystis aeruginosa PCC 7806.Curr Genet. 2016 Nov;62(4):669-675. doi: 10.1007/s00294-016-0582-6. Epub 2016 Mar 11. Curr Genet. 2016. PMID: 26968707 Review.
-
Orthocaspases are proteolytically active prokaryotic caspase homologues: the case of Microcystis aeruginosa.Mol Microbiol. 2015 Oct;98(1):142-50. doi: 10.1111/mmi.13110. Epub 2015 Jul 17. Mol Microbiol. 2015. PMID: 26114948
-
The art of destruction: revealing the proteolytic capacity of bacterial caspase homologs.Mol Microbiol. 2015 Oct;98(1):1-6. doi: 10.1111/mmi.13111. Epub 2015 Jul 22. Mol Microbiol. 2015. PMID: 26123017
-
ClpAP protease is a universal factor that activates the parDE toxin-antitoxin system from a broad host range RK2 plasmid.Sci Rep. 2018 Oct 16;8(1):15287. doi: 10.1038/s41598-018-33726-y. Sci Rep. 2018. PMID: 30327496 Free PMC article.
-
Keeping the Wolves at Bay: Antitoxins of Prokaryotic Type II Toxin-Antitoxin Systems.Front Mol Biosci. 2016 Mar 22;3:9. doi: 10.3389/fmolb.2016.00009. eCollection 2016. Front Mol Biosci. 2016. PMID: 27047942 Free PMC article. Review.
Cited by
-
AliC and AliD of nonencapsulated Streptococcus pneumoniae enhance virulence in a Galleria mellonella model of infection by contributing to reactive oxygen species resistance.Front Cell Infect Microbiol. 2025 Jun 11;15:1583375. doi: 10.3389/fcimb.2025.1583375. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40568701 Free PMC article.
-
Friend or Foe: Protein Inhibitors of DNA Gyrase.Biology (Basel). 2024 Jan 29;13(2):84. doi: 10.3390/biology13020084. Biology (Basel). 2024. PMID: 38392303 Free PMC article. Review.
-
Conformational change as a mechanism for toxin activation in bacterial toxin-antitoxin systems.J Virol. 2024 Nov 19;98(11):e0151324. doi: 10.1128/jvi.01513-24. Epub 2024 Oct 24. J Virol. 2024. PMID: 39445801 Free PMC article. Review.
-
Control of Toxin-Antitoxin Systems by Proteases in Mycobacterium Tuberculosis.Front Mol Biosci. 2021 May 17;8:691399. doi: 10.3389/fmolb.2021.691399. eCollection 2021. Front Mol Biosci. 2021. PMID: 34079824 Free PMC article. Review.
-
Structural insights into the PrpTA toxin-antitoxin system in Pseudoalteromonas rubra.Front Microbiol. 2022 Nov 24;13:1053255. doi: 10.3389/fmicb.2022.1053255. eCollection 2022. Front Microbiol. 2022. PMID: 36504814 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

