The Cholinergic System Contributes to the Immunopathological Progression of Experimental Pulmonary Tuberculosis
- PMID: 33679685
- PMCID: PMC7930380
- DOI: 10.3389/fimmu.2020.581911
The Cholinergic System Contributes to the Immunopathological Progression of Experimental Pulmonary Tuberculosis
Abstract
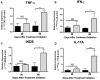
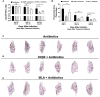
The cholinergic system is present in both bacteria and mammals and regulates inflammation during bacterial respiratory infections through neuronal and non-neuronal production of acetylcholine (ACh) and its receptors. However, the presence of this system during the immunopathogenesis of pulmonary tuberculosis (TB) in vivo and in its causative agent Mycobacterium tuberculosis (Mtb) has not been studied. Therefore, we used an experimental model of progressive pulmonary TB in BALB/c mice to quantify pulmonary ACh using high-performance liquid chromatography during the course of the disease. In addition, we performed immunohistochemistry in lung tissue to determine the cellular expression of cholinergic system components, and then administered nicotinic receptor (nAChR) antagonists to validate their effect on lung bacterial burden, inflammation, and pro-inflammatory cytokines. Finally, we subjected Mtb cultures to colorimetric analysis to reveal the production of ACh and the effect of ACh and nAChR antagonists on Mtb growth. Our results show high concentrations of ACh and expression of its synthesizing enzyme choline acetyltransferase (ChAT) during early infection in lung epithelial cells and macrophages. During late progressive TB, lung ACh upregulation was even higher and coincided with ChAT and α7 nAChR subunit expression in immune cells. Moreover, the administration of nAChR antagonists increased pro-inflammatory cytokines, reduced bacillary loads and synergized with antibiotic therapy in multidrug resistant TB. Finally, in vitro studies revealed that the bacteria is capable of producing nanomolar concentrations of ACh in liquid culture. In addition, the administration of ACh and nicotinic antagonists to Mtb cultures induced or inhibited bacterial proliferation, respectively. These results suggest that Mtb possesses a cholinergic system and upregulates the lung non-neuronal cholinergic system, particularly during late progressive TB. The upregulation of the cholinergic system during infection could aid both bacterial growth and immunomodulation within the lung to favor disease progression. Furthermore, the therapeutic efficacy of modulating this system suggests that it could be a target for treating the disease.
Keywords: acetylcholine; choline acetyltransferase; cholinergic; immune response; mycobacterium tuberculosis; nAChR antagonism; pulmonary inflammation; tuberculosis.
Copyright © 2021 Islas-Weinstein, Marquina-Castillo, Mata-Espinosa, Paredes-González, Chávez, Balboa, Marín Franco, Guerrero-Romero, Barrios-Payan and Hernandez-Pando.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

