A Novel Anti-HER2 Bispecific Antibody With Potent Tumor Inhibitory Effects In Vitro and In Vivo
- PMID: 33679691
- PMCID: PMC7927792
- DOI: 10.3389/fimmu.2020.600883
A Novel Anti-HER2 Bispecific Antibody With Potent Tumor Inhibitory Effects In Vitro and In Vivo
Abstract
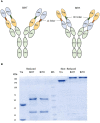
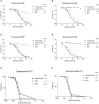
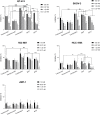
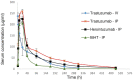
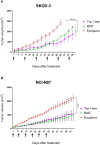
Overexpression of HER2 has been reported in many types of cancer, making it a perfect candidate for targeted immunotherapy. The combination of two FDA approved monoclonal antibodies (mAbs), trastuzumab and pertuzumab, has more robust anti-tumor activity in patients with HER2-overexpressing breast cancer. We recently produced a new humanized anti-HER2 mAb, hersintuzumab, which recognizes a different epitope than trastuzumab and pertuzumab on HER2. This mAb, in combination with trastuzumab, exhibits more potent anti-tumor activity than each parental mAb alone. Here we have developed a novel bispecific anti-HER2 antibody (BsAb) designated as trasintuzumab, composed of trastuzumab and hersintuzumab, using dual variable domain immunoglobulin (DVD-Ig) technology. Both variable domains of trasintuzumab are fully functional and have similar affinities to the parental mAbs and are also able to bind to natural HER2 on the surface of several HER2-expressing cell lines. Trasintuzumab was found to inhibit the growth of different types of tumor cell lines through suppression of the AKT and ERK signaling pathways as efficiently as the combination of the parental mAbs. It also induced tumor regression as potently as the combination of the two mAbs in nude mice bearing ovarian and gastric cancer xenografts. Our data suggest that trasintuzumab may be a promising BsAb therapeutic candidate for the treatment of HER2-overexpressing cancers.
Keywords: DVD-Ig; HER2; bispecific antibody; cancer immunotherapy; monoclonal antibody.
Copyright © 2021 Mohammadi, Jeddi-Tehrani, Golsaz-Shirazi, Arjmand, Bahadori, Judaki, Shiravi, Zare, Haghighat, Mobini, Amiri and Shokri.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Potent synergistic anti-tumor activity of a novel humanized anti-HER2 antibody hersintuzumab in combination with trastuzumab in xenograft models.Invest New Drugs. 2021 Jun;39(3):697-704. doi: 10.1007/s10637-020-01048-4. Epub 2021 Jan 3. Invest New Drugs. 2021. PMID: 33389387
-
Structural and functional characterization of MBS301, an afucosylated bispecific anti-HER2 antibody.MAbs. 2018 Aug/Sep;10(6):864-875. doi: 10.1080/19420862.2018.1486946. Epub 2018 Aug 6. MAbs. 2018. PMID: 30081724 Free PMC article.
-
Hersintuzumab: A novel humanized anti-HER2 monoclonal antibody induces potent tumor growth inhibition.Invest New Drugs. 2018 Apr;36(2):171-186. doi: 10.1007/s10637-017-0518-0. Epub 2017 Oct 6. Invest New Drugs. 2018. PMID: 28983766
-
Mechanism of action of anti-HER2 monoclonal antibodies: scientific update on trastuzumab and 2C4.Adv Exp Med Biol. 2003;532:253-68. doi: 10.1007/978-1-4615-0081-0_21. Adv Exp Med Biol. 2003. PMID: 12908564 Review.
-
Monoclonal Antibodies in Clinical Trials for Breast Cancer Treatment.Monoclon Antib Immunodiagn Immunother. 2025 Apr;44(2):17-39. doi: 10.1089/mab.2024.0018. Epub 2025 Apr 2. Monoclon Antib Immunodiagn Immunother. 2025. PMID: 40171653 Review.
Cited by
-
Anti-HER2 biparatopic antibody KJ015 has near-native structure, functional balanced high affinity, and synergistic efficacy with anti-PD-1 treatment in vivo.MAbs. 2024 Jan-Dec;16(1):2412881. doi: 10.1080/19420862.2024.2412881. Epub 2024 Oct 9. MAbs. 2024. PMID: 39381966 Free PMC article.
-
Clinical implication of genetic composition and molecular mechanism on treatment strategies of HER2-positive breast cancers.Front Oncol. 2022 Oct 31;12:964824. doi: 10.3389/fonc.2022.964824. eCollection 2022. Front Oncol. 2022. PMID: 36387174 Free PMC article. Review.
-
A novel nanobody-based HER2-targeting antibody exhibits potent synergistic antitumor efficacy in trastuzumab-resistant cancer cells.Front Immunol. 2023 Oct 25;14:1292839. doi: 10.3389/fimmu.2023.1292839. eCollection 2023. Front Immunol. 2023. PMID: 37954614 Free PMC article.
-
From Anti-HER-2 to Anti-HER-2-CAR-T Cells: An Evolutionary Immunotherapy Approach for Gastric Cancer.J Inflamm Res. 2022 Jul 17;15:4061-4085. doi: 10.2147/JIR.S368138. eCollection 2022. J Inflamm Res. 2022. PMID: 35873388 Free PMC article. Review.
-
Hyperthermia in Combination with Emerging Targeted and Immunotherapies as a New Approach in Cancer Treatment.Cancers (Basel). 2024 Jan 24;16(3):505. doi: 10.3390/cancers16030505. Cancers (Basel). 2024. PMID: 38339258 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

