Trained Immunity-Based Vaccine in B Cell Hematological Malignancies With Recurrent Infections: A New Therapeutic Approach
- PMID: 33679698
- PMCID: PMC7928395
- DOI: 10.3389/fimmu.2020.611566
Trained Immunity-Based Vaccine in B Cell Hematological Malignancies With Recurrent Infections: A New Therapeutic Approach
Abstract
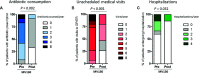
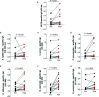
Infectious complications are a major cause of morbidity and mortality in B-cell hematological malignancies (HM). Prophylaxis for recurrent infections in HM patients with antibody deficiency consists of first-line antibiotics and when unsuccessful, gammaglobulin replacement therapy (IgRT). Recent knowledge of trained immunity-based vaccines (TIbV), such as the sublingual polybacterial formulation MV130, has shown a promising strategy in the management of patients with recurrent infections. We sought to determine the clinical benefit of MV130 in a cohort of HM patients with recurrent respiratory tract infections (RRTIs) who underwent immunization with MV130 for 3 months. Clinical information included the frequency of infections, antibiotic use, number of visits to the GP and hospitalizations previous and after MV130 immunotherapy. Improvement on infection rate was classified as: clear (>60% reduction of infection), partial (26%-60%) and low (≤25%) improvement. Fifteen HM patients (aged 42 to 80 years; nine females) were included in the study. All patients reduced their infection rate. Analysis of paired data revealed that the median (range, min - max) of respiratory infectious rate significantly decreased from 4.0 (8.0-3.0) to 2.0 (4.0-0.0) (p<0.001) at 12 months of MV130. A clear clinical improvement was observed in 53% (n = 8) of patients, partial improvement in 40% (n = 6) and low improvement in 7% (n = 1). These data correlated with a decrease on antibiotic consumption from 3.0 (8.0-1.0) to 1.0 (2.0-0.0) (p = 0.002) during 12 months after initiation of treatment with MV130. The number of infectious-related GP or emergency room visits declined from 4.0 (8.0-2.0) to 2.0 (3.0-0.0) (p<0.001), in parallel with a reduction in hospital admissions due to infections (p = 0.032). Regarding safety, no adverse events were observed. On the other hand, immunological assessment of serum IgA and IgG levels demonstrated an increase in specific antibodies to MV130-contained bacteria following MV130 immunotherapy. In conclusion, MV130 may add clinical benefit reducing the rate of infections and enhancing humoral immune responses in these vulnerable patients.
Keywords: IgA; MV130; hematological malignancies; prophylaxis; recurrent respiratory tract infections; trained immunity-based vaccines.
Copyright © 2021 Ochoa-Grullón, Benavente Cuesta, González Fernández, Cordero Torres, Pérez López, Peña Cortijo, Conejero Hall, Mateo Morales, Rodríguez de la Peña, Díez-Rivero, Rodríguez de Frías, Guevara-Hoyer, Fernández-Arquero and Sánchez-Ramón.
Conflict of interest statement
LC and CD-R belong to the Immunotek R+D Department. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

