Cancer Vaccines: Adjuvant Potency, Importance of Age, Lifestyle, and Treatments
- PMID: 33679703
- PMCID: PMC7927599
- DOI: 10.3389/fimmu.2020.615240
Cancer Vaccines: Adjuvant Potency, Importance of Age, Lifestyle, and Treatments
Abstract
Although the discovery and characterization of multiple tumor antigens have sparked the development of many antigen/derived cancer vaccines, many are poorly immunogenic and thus, lack clinical efficacy. Adjuvants are therefore incorporated into vaccine formulations to trigger strong and long-lasting immune responses. Adjuvants have generally been classified into two categories: those that 'depot' antigens (e.g. mineral salts such as aluminum hydroxide, emulsions, liposomes) and those that act as immunostimulants (Toll Like Receptor agonists, saponins, cytokines). In addition, several novel technologies using vector-based delivery of antigens have been used. Unfortunately, the immune system declines with age, a phenomenon known as immunosenescence, and this is characterized by functional changes in both innate and adaptive cellular immunity systems as well as in lymph node architecture. While many of the immune functions decline over time, others paradoxically increase. Indeed, aging is known to be associated with a low level of chronic inflammation-inflamm-aging. Given that the median age of cancer diagnosis is 66 years and that immunotherapeutic interventions such as cancer vaccines are currently given in combination with or after other forms of treatments which themselves have immune-modulating potential such as surgery, chemotherapy and radiotherapy, the choice of adjuvants requires careful consideration in order to achieve the maximum immune response in a compromised environment. In addition, more clinical trials need to be performed to carefully assess how less conventional form of immune adjuvants, such as exercise, diet and psychological care which have all be shown to influence immune responses can be incorporated to improve the efficacy of cancer vaccines. In this review, adjuvants will be discussed with respect to the above-mentioned important elements.
Keywords: adjuvant; cancer vaccine; immunosenescence; immunotherapy; inflamm-aging; microbiota.
Copyright © 2021 Cuzzubbo, Mangsbo, Nagarajan, Habra, Pockley and McArdle.
Conflict of interest statement
SMa is the Chief Development Officer at Ultimovacs AB, a company that develops cancer vaccines and holds patent applications within the field of cancer vaccines. SMa is also the founder of Immuneed AB and Vivologica AB. AP is the Chief Executive Officer of multimmune GmbH, a company that develops cancer “theranostics” based on tumor expression of membrane Hsp70. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
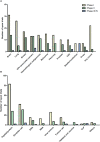
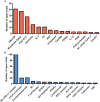

References
-
- Lawson DH, Lee S, Zhao F, Tarhini AA, Margolin KA, Ernstoff MS, et al. Randomized, Placebo-Controlled, Phase III Trial of Yeast-Derived Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) Versus Peptide Vaccination Versus GM-CSF Plus Peptide Vaccination Versus Placebo in Patients With No Evidence of Disease After Complete Surgical Resection of Locally Advanced and/or Stage IV Melanoma: A Trial of the Eastern Cooperative Oncology Group-American College of Radiology Imaging Network Cancer Research Group (E4697). J Clin Oncol (2015) 33(34):4066–76. 10.1200/JCO.2015.62.0500 - DOI - PMC - PubMed
-
- Dreno B, Thompson JF, Smithers BM, Santinami M, Jouary T, Gutzmer R, et al. MAGE-A3 immunotherapeutic as adjuvant therapy for patients with resected, MAGE-A3-positive, stage III melanoma (DERMA): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol (2018) 19(7):916–29. 10.1016/S1470-2045(18)30254-7 - DOI - PubMed
-
- Eggermont AM, Suciu S, Ruka W, Marsden J, Testori A, Corrie P, et al. EORTC 18961: Post-operative adjuvant ganglioside GM2-KLH21 vaccination treatment vs observation in stage II (T3-T4N0M0) melanoma: 2nd interim analysis led to an early disclosure of the results. [Abstract]. J Clin Oncol (2008) 26(Suppl 15):A–9004. 10.1200/jco.2008.26.15_suppl.9004 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

