β 2 Integrin Signaling Cascade in Neutrophils: More Than a Single Function
- PMID: 33679708
- PMCID: PMC7930317
- DOI: 10.3389/fimmu.2020.619925
β 2 Integrin Signaling Cascade in Neutrophils: More Than a Single Function
Abstract
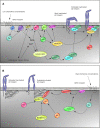
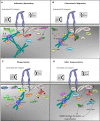
Neutrophils are the most prevalent leukocytes in the human body. They have a pivotal role in the innate immune response against invading bacterial and fungal pathogens, while recent emerging evidence also demonstrates their role in cancer progression and anti-tumor responses. The efficient execution of many neutrophil effector responses requires the presence of β2 integrins, in particular CD11a/CD18 or CD11b/CD18 heterodimers. Although extensively studied at the molecular level, the exact signaling cascades downstream of β2 integrins still remain to be fully elucidated. In this review, we focus mainly on inside-out and outside-in signaling of these two β2 integrin members expressed on neutrophils and describe differences between various neutrophil stimuli with respect to integrin activation, integrin ligand binding, and the pertinent differences between mouse and human studies. Last, we discuss how integrin signaling studies could be used to explore the therapeutic potential of targeting β2 integrins and the intracellular signaling cascade in neutrophils in several, among other, inflammatory conditions in which neutrophil activity should be dampened to mitigate disease.
Keywords: CD11b/CD18 integrin; neutrophil function; neutrophils; therapeutic targets; β2 integrin signaling.
Copyright © 2021 Bouti, Webbers, Fagerholm, Alon, Moser, Matlung and Kuijpers.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

