Evolving Antibody Therapies for the Treatment of Type 1 Diabetes
- PMID: 33679717
- PMCID: PMC7930374
- DOI: 10.3389/fimmu.2020.624568
Evolving Antibody Therapies for the Treatment of Type 1 Diabetes
Abstract
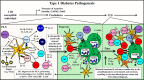
Type 1 diabetes (T1D) is widely considered to be a T cell driven autoimmune disease resulting in reduced insulin production due to dysfunction/destruction of pancreatic β cells. Currently, there continues to be a need for immunotherapies that selectively reestablish persistent β cell-specific self-tolerance for the prevention and remission of T1D in the clinic. The utilization of monoclonal antibodies (mAb) is one strategy to target specific immune cell populations inducing autoimmune-driven pathology. Several mAb have proven to be clinically safe and exhibit varying degrees of efficacy in modulating autoimmunity, including T1D. Traditionally, mAb therapies have been used to deplete a targeted cell population regardless of antigenic specificity. However, this treatment strategy can prove detrimental resulting in the loss of acquired protective immunity. Nondepleting mAb have also been applied to modulate the function of immune effector cells. Recent studies have begun to define novel mechanisms associated with mAb-based immunotherapy that alter the function of targeted effector cell pools. These results suggest short course mAb therapies may have persistent effects for regaining and maintaining self-tolerance. Furthermore, the flexibility to manipulate mAb properties permits the development of novel strategies to target multiple antigens and/or deliver therapeutic drugs by a single mAb molecule. Here, we discuss current and potential future therapeutic mAb treatment strategies for T1D, and T cell-mediated autoimmunity.
Keywords: diabetes; immunoregulation; immunotherapy; monoclonal antibodies; self-tolerance.
Copyright © 2021 Ke, Kroger, Clark and Tisch.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Eisenbarth GS. Type 1 diabetes: molecular, cellular and clinical immunology. Adv Exp Med Biol (2004) 552:306–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

