Pathogenicity of Proteinase 3-Anti-Neutrophil Cytoplasmic Antibody in Granulomatosis With Polyangiitis: Implications as Biomarker and Future Therapies
- PMID: 33679731
- PMCID: PMC7930335
- DOI: 10.3389/fimmu.2021.571933
Pathogenicity of Proteinase 3-Anti-Neutrophil Cytoplasmic Antibody in Granulomatosis With Polyangiitis: Implications as Biomarker and Future Therapies
Abstract
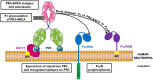
Granulomatosis with polyangiitis (GPA) is a rare but serious necrotizing auto-immune vasculitis. GPA is mostly associated with the presence of Anti-Neutrophil Cytoplasmic Antibody (ANCA) targeting proteinase 3 (PR3-ANCA), a serine protease contained in neutrophil granules but also exposed at the membrane. PR3-ANCAs have a proven fundamental role in GPA: they bind neutrophils allowing their auto-immune activation responsible for vasculitis lesions. PR3-ANCAs bind neutrophil surface on the one hand by their Fab binding PR3 and on the other by their Fc binding Fc gamma receptors. Despite current therapies, GPA is still a serious disease with an important mortality and a high risk of relapse. Furthermore, although PR3-ANCAs are a consistent biomarker for GPA diagnosis, relapse management currently based on their level is inconsistent. Indeed, PR3-ANCA level is not correlated with disease activity in 25% of patients suggesting that not all PR3-ANCAs are pathogenic. Therefore, the development of new biomarkers to evaluate disease activity and predict relapse and new therapies is necessary. Understanding factors influencing PR3-ANCA pathogenicity, i.e. their potential to induce auto-immune activation of neutrophils, offers interesting perspectives in order to improve GPA management. Most relevant factors influencing PR3-ANCA pathogenicity are involved in their interaction with neutrophils: level of PR3 autoantigen at neutrophil surface, epitope of PR3 recognized by PR3-ANCA, isotype and glycosylation of PR3-ANCA. We detailed in this review the advances in understanding these factors influencing PR3-ANCA pathogenicity in order to use them as biomarkers and develop new therapies in GPA as part of a personalized approach.
Keywords: anti-neutrophil cytoplasmic antibodies; biomarkers; granulomatosis with polyangiitis; human neutrophils; new therapies; pathogenicity; proteinase 3.
Copyright © 2021 Granel, Korkmaz, Nouar, Weiss, Jenne, Lemoine and Hoarau.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Bossuyt X, Cohen Tervaert J-W, Arimura Y, Blockmans D, Flores-Suárez LF, Guillevin L, et al. Position paper: Revised 2017 international consensus on testing of ANCAs in granulomatosis with polyangiitis and microscopic polyangiitis. Nat Rev Rheumatol (2017) 13(11):683−92. 10.1038/nrrheum.2017.140 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

