The "Gum-Gut" Axis in Inflammatory Bowel Diseases: A Hypothesis-Driven Review of Associations and Advances
- PMID: 33679761
- PMCID: PMC7933581
- DOI: 10.3389/fimmu.2021.620124
The "Gum-Gut" Axis in Inflammatory Bowel Diseases: A Hypothesis-Driven Review of Associations and Advances
Abstract
In modern medicine, the oral cavity has often been viewed as a passive conduit to the upper airways and gastrointestinal tract; however, its connection to the rest of the body has been increasingly explored over the last 40 years. For several diseases, the periodontium and gingiva are at the center of this oral-systemic link. Over 50 systemic conditions have been specifically associated with gingival and periodontal inflammation, including inflammatory bowel diseases (IBD), which have recently been elevated from simple "associations" to elegant, mechanistic investigations. IBD and periodontitis have been reported to impact each other's progression via a bidirectional relationship whereby chronic oral or intestinal inflammation can impact the other; however, the precise mechanisms for how this occurs remain unclear. Classically, the etiology of gingival inflammation (gingivitis) is oral microbial dysbiosis in the subgingival crevice that can lead to destructive periodontal disease (periodontitis); however, the current understanding of gingival involvement in IBD is that it may represent a separate disease entity from classical gingivitis, arising from mechanisms related to systemic inflammatory activation of niche-resident immune cells. Synthesizing available evidence, we hypothesize that once established, IBD can be driven by microbiomial and inflammatory changes originating specifically from the gingival niche through saliva, thereby worsening IBD outcomes and thus perpetuating a vicious cycle. In this review, we introduce the concept of the "gum-gut axis" as a framework for examining this reciprocal relationship between the periodontium and the gastrointestinal tract. To support and explore this gum-gut axis, we 1) provide a narrative review of historical studies reporting gingival and periodontal manifestations in IBD, 2) describe the current understanding and advances for the gum-gut axis, and 3) underscore the importance of collaborative treatment and research plans between oral and GI practitioners to benefit this patient population.
Keywords: Crohn’s disease; gingivitis; gum–gut; inflammatory bowel disease; microbiome; oral–gut; periodontitis; ulcerative colitis.
Copyright © 2021 Byrd and Gulati.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

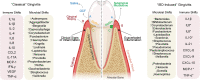



References
-
- Nanci A, Ten CAR. Ten Cate's Oral Histology: Development, Structure, and Function. St. Louis, Mo: Mosby; (2017).
-
- Regezi JA, Sciubba JJ, Jordan RC. Oral pathology: clinical pathologic correlations. 6th Ed, St. Louis: Elsevier Saunders; (2016) 388.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

