Exploring the Role of ATP-Citrate Lyase in the Immune System
- PMID: 33679780
- PMCID: PMC7930476
- DOI: 10.3389/fimmu.2021.632526
Exploring the Role of ATP-Citrate Lyase in the Immune System
Abstract
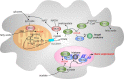
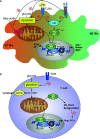
Studies over the past decade have revealed that metabolism profoundly influences immune responses. In particular, metabolism causes epigenetic regulation of gene expression, as a growing number of metabolic intermediates are substrates for histone post-translational modifications altering chromatin structure. One of these substrates is acetyl-coenzyme A (CoA), which donates an acetyl group for histone acetylation. Cytosolic acetyl-CoA is also a critical substrate for de novo synthesis of fatty acids and sterols necessary for rapid cellular growth. One of the main enzymes catalyzing cytosolic acetyl-CoA formation is ATP-citrate lyase (ACLY). In addition to its classical function in the provision of acetyl-CoA for de novo lipogenesis, ACLY contributes to epigenetic regulation through histone acetylation, which is increasingly appreciated. In this review we explore the current knowledge of ACLY and acetyl-CoA in mediating innate and adaptive immune responses. We focus on the role of ACLY in supporting de novo lipogenesis in immune cells as well as on its impact on epigenetic alterations. Moreover, we summarize alternative sources of acetyl-CoA and their contribution to metabolic and epigenetic regulation in cells of the immune system.
Keywords: ATP-citrate lyase; acetyl-CoA; histone acetylation; macrophage; metabolism.
Copyright © 2021 Dominguez, Brüne and Namgaladze.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

