Inhibition of NLRP3 Inflammasome Activation and Pyroptosis in Macrophages by Taraxasterol Is Associated With Its Regulation on mTOR Signaling
- PMID: 33679781
- PMCID: PMC7925414
- DOI: 10.3389/fimmu.2021.632606
Inhibition of NLRP3 Inflammasome Activation and Pyroptosis in Macrophages by Taraxasterol Is Associated With Its Regulation on mTOR Signaling
Abstract
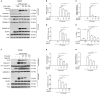
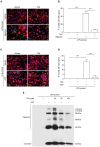
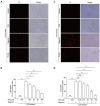
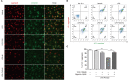
Taraxasterol (TAS) is an active ingredient of Dandelion (Taraxacum mongolicum Hand. -Mazz.), a medicinal plant that has long been used in China for treatment of inflammatory disorders. But the underlying mechanism for its therapeutic effects on inflammatory disorders is not completely clear. Inflammasome activation is a critical step of innate immune response to infection and aseptic inflammation. Among the various types of inflammasome sensors that has been reported, NLR family pyrin domain containing 3 (NLRP3) is implicated in various inflammatory diseases and therefore has been most extensively studied. In this study, we aimed to explore whether TAS could influence NLPR3 inflammasome activation in macrophages. The results showed that TAS dose-dependently suppressed the activation of caspase-1 in lipopolysaccharide (LPS)-primed murine primary macrophages upon nigericin treatment, resulting in reduced mature interleukin-1β (IL-1β) release and gasdermin D (GSDMD) cleavage. TAS greatly reduced ASC speck formation upon the stimulation of nigericin or extracellular ATP. Consistent with reduced cleavage of GSDMD, nigericin-induced pyroptosis was alleviated by TAS. Interestingly, TAS time-dependently suppressed the mechanistic target of rapamycin (mTOR) complex 1 (mTORC1) and mTORC2 signaling induced by LPS priming. Like TAS, both INK-128 (inhibiting both mTORC1 and mTORC2) and rapamycin (inhibiting mTORC1 only) also inhibited NLRP3 inflammasome activation, though their effects on mTOR signaling were different. Moreover, TAS treatment alleviated mitochondrial damage by nigericin and improved mouse survival from bacterial infection, accompanied by reduced IL-1β levels in vivo. Collectively, by inhibiting the NLRP3 inflammasome activation, TAS displayed anti-inflammatory effects likely through regulation of the mTOR signaling in macrophages, highlighting a potential action mechanism for the anti-inflammatory activity of Dandelion in treating inflammation-related disorders, which warrants further clinical investigation.
Keywords: ASC speck; NLRP3 inflammasome; dandelion; mTOR; taraxasterol.
Copyright © 2021 Yang, Ye, Chen, Li, Wang, Zhong, Zhong, Zeng, Xu, He and Ouyang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

