Preparation, Statistical Optimization and In-vitro Characterization of a Dry Powder Inhaler (DPI) Containing Solid Lipid Nanoparticles Encapsulating Amphotericin B: Ion Paired Complexes with Distearoyl Phosphatidylglycerol
- PMID: 33680009
- PMCID: PMC7757990
- DOI: 10.22037/ijpr.2019.15208.12963
Preparation, Statistical Optimization and In-vitro Characterization of a Dry Powder Inhaler (DPI) Containing Solid Lipid Nanoparticles Encapsulating Amphotericin B: Ion Paired Complexes with Distearoyl Phosphatidylglycerol
Abstract
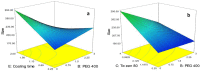
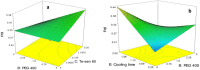
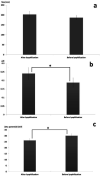
The aim of this study was to prepare dry powder inhalers (DPIs) containing amphotericin B-loaded solid lipid nanoparticles (AMB-SLNs) as an alternative approach for prevention of pulmonary aspergillosis. For solubilizing AMB in small amounts of organic solvents ion paired complexes were firstly formed by establishing electrostatic interaction between AMB and distearoyl phosphatidylglycerol (DSPG). The SLN formulations containing AMB-DSPG complexes were prepared using glycerol monostearate (GMS) as the lipid matrix and soybean lecithin and tween 80 as the surfactants by solvent emulsification-evaporation technique. The nanoparticles were optimized through a fractional factorial design. DPIs were prepared by lyophilization technique using lactose as the inhalational carrier and then after, the formulations were evaluated in terms of aerodynamic particle size distribution using an Andersen cascade impactor. The morphology of the particles was examined using scanning electron microscopy (SEM) and in-vitro drug release profiles were evaluated. Following the statistical results, the particle size, Poly dispersity index (PdI), zeta potential, entrapment efficiency (EE%), and drug loading (DL%) of the optimized SLNs were 187.04 ± 11.97 nm, 0.188 ± 0.028, -30.16 ± 1.6 mV, 89.3 ± 3.47 % and 2.76 ± 0.32 %, respectively. Formulation containing 10% w/v of lactose with the calculated fine particle fraction value as 72.57 ± 4.33% exhibited the appropriate aerodynamic characteristics for pulmonary drug delivery. SEM images revealed de-agglomerated particles. In-vitro release studies showed sustained release of AMB from the carriers and the release kinetics were best fitted to the first order kinetic model.
Keywords: Amphotericin B; Distearoyl phosphatidylglycerol (DSPG); Dry powder inhaler (DPI); Ion paired complexation; Lyophilization technique; Solid lipid nanoparticle (SLN).
Figures



Similar articles
-
Preparation, Statistical Optimization and Characterization of Propolis-Loaded Solid Lipid Nanoparticles Using Box-Behnken Design.Adv Pharm Bull. 2021 Feb;11(2):301-310. doi: 10.34172/apb.2021.043. Epub 2020 Jun 20. Adv Pharm Bull. 2021. PMID: 33880352 Free PMC article.
-
Preparation of Curcumin Solid Lipid Nanoparticles Loaded with Flower-Shaped Lactose for Lung Inhalation and Preliminary Evaluation of Cytotoxicity In Vitro.Evid Based Complement Alternat Med. 2021 Oct 28;2021:4828169. doi: 10.1155/2021/4828169. eCollection 2021. Evid Based Complement Alternat Med. 2021. Retraction in: Evid Based Complement Alternat Med. 2023 Dec 13;2023:9809240. doi: 10.1155/2023/9809240. PMID: 34745284 Free PMC article. Retracted.
-
Amphotericin B-loaded solid lipid nanoparticles (SLNs) and nanostructured lipid carrier (NLCs): effect of drug loading and biopharmaceutical characterizations.Drug Dev Ind Pharm. 2018 Oct;44(10):1693-1700. doi: 10.1080/03639045.2018.1492606. Epub 2018 Jul 23. Drug Dev Ind Pharm. 2018. PMID: 29936874
-
Potential and constraints for the application of CFD combined with Lagrangian particle tracking to dry powder inhalers.Eur J Pharm Sci. 2019 Feb 1;128:299-324. doi: 10.1016/j.ejps.2018.12.008. Epub 2018 Dec 14. Eur J Pharm Sci. 2019. PMID: 30553814 Review.
-
Influence of physical properties of carrier on the performance of dry powder inhalers.Acta Pharm Sin B. 2016 Jul;6(4):308-18. doi: 10.1016/j.apsb.2016.03.011. Epub 2016 May 4. Acta Pharm Sin B. 2016. PMID: 27471671 Free PMC article. Review.
Cited by
-
Could the Lung Be a Gateway for Amphotericin B to Attack the Army of Fungi?Pharmaceutics. 2022 Dec 3;14(12):2707. doi: 10.3390/pharmaceutics14122707. Pharmaceutics. 2022. PMID: 36559201 Free PMC article. Review.
-
Inhalable Nanotechnology-Based Drug Delivery Systems for the Treatment of Inflammatory Lung Diseases.Pharmaceutics. 2025 Jul 9;17(7):893. doi: 10.3390/pharmaceutics17070893. Pharmaceutics. 2025. PMID: 40733101 Free PMC article. Review.
-
Unroasted Green Coffee Extract-Loaded Solid Lipid Nanoparticles for Enhancing Intestinal Permeation.ACS Omega. 2023 Jun 1;8(23):20251-20261. doi: 10.1021/acsomega.2c06629. eCollection 2023 Jun 13. ACS Omega. 2023. PMID: 37332788 Free PMC article.
-
Inhaled Antifungal Agents for Treatment and Prophylaxis of Bronchopulmonary Invasive Mold Infections.Pharmaceutics. 2022 Mar 14;14(3):641. doi: 10.3390/pharmaceutics14030641. Pharmaceutics. 2022. PMID: 35336015 Free PMC article. Review.
-
Anti-CD44 and EGFR Dual-Targeted Solid Lipid Nanoparticles for Delivery of Doxorubicin to Triple-Negative Breast Cancer Cell Line: Preparation, Statistical Optimization, and In Vitro Characterization.Biomed Res Int. 2022 Jul 6;2022:6253978. doi: 10.1155/2022/6253978. eCollection 2022. Biomed Res Int. 2022. PMID: 35845934 Free PMC article.
References
-
- Zhou QT, Leung SS, Tang P, Parumasivam T, Loh ZH, Chan HK. Inhaled formulations and pulmonary drug delivery systems for respiratory infections. Adv. Drug. Deliv. Rev. . 2015;85:83–99. - PubMed
-
- Bulpa P, Dive A, Sibille Y. Invasive pulmonary aspergillosis in patients with chronic obstructive pulmonary disease. Eur. Respir. J. . 2007;30:782–800. - PubMed
-
- Salama A, Burger M, Mueller Eckhardt C. Acute immune hemolysis induced by a degradation product of Amphotericin B. Blut. . 1989;58:59–61. - PubMed
-
- Wang LH, Smith PC, Andersen KL. High- performance liquid chromatographic analysis of amphotericin B in plasma, blood urine and tissues for pharmacokinetic and tissue distribution studies. J. Chromatogr. . 1992;579:259–68. - PubMed
-
- Fanos V, Cataldi L. Amphotericin B-induced nephrotoxicity: A review. J. Chemother. . 2000;12:463–70. - PubMed
LinkOut - more resources
Full Text Sources
