Long non-coding RNA SNHG7 facilitates pancreatic cancer progression by regulating the miR-146b-5p/Robo1 axis
- PMID: 33680120
- PMCID: PMC7918173
- DOI: 10.3892/etm.2021.9829
Long non-coding RNA SNHG7 facilitates pancreatic cancer progression by regulating the miR-146b-5p/Robo1 axis
Abstract
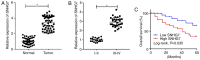
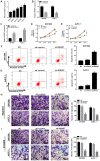
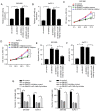
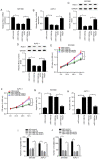
Long non-coding RNA (lncRNA) small nucleolar RNA host gene 7 (SNHG7) plays a crucial role in the progression of pancreatic cancer (PC). SNHG7 is upregulated in PC; therefore, the purpose of the present study was to investigate the role and underlying mechanism of SNHG7 on PC progression. In the present study, the mRNA expression levels of SNHG7, microRNA(miR)-146b-5p and roundabout homolog 1 (Robo1) were measured via reverse transcription-quantitative PCR. Moreover, cell viability and apoptosis were assessed by MTT and flow cytometry assays, respectively. The ability of cells to migrate and invade was evaluated by Transwell assays. In addition, dual-luciferase reporter, RNA immunoprecipitation and RNA pull-down assays were conducted to assess the interaction between miR-146b-5p and SNHG7 or Robo1. The protein expression of Robo1 was measured via western blotting. Furthermore, mouse xenograft models were established to further investigate the effect of SNHG7 on PC progression in vivo. The results indicated that SNHG7 was highly expressed in PC tissues and cells. It was also found that SNHG7 was sponged by miR-146b-5p and that Robo1 was a target of miR-146b-5p. Moreover, it was demonstrated that SNHG7 knockdown inhibited cell proliferation, migration and invasion, as well as tumorigenesis and apoptosis of PC cells in vitro and in vivo by regulating miR-146b-5p. The results also suggested that miR-146b-5p overexpression inhibited the progression of PC cells by modulating Robo1. Furthermore, silencing of SNHG7 downregulated Robo1 expression by sponging miR-146b-5p. Collectively, the present results indicate that SNHG7 promotes PC progression by sponging miR-146b-5p and upregulating Robo1.
Keywords: cancer progression; long non-coding RNA small nucleolar RNA host gene 7; microRNA-146b-5p; pancreatic cancer; roundabout homolog 1.
Copyright: © Jian et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Surveillance Epidemiology and End Results (SEER) Fact Sheets: Pancreas. Web site. http://seer.cancer.gov/. Accessed July 15, 2016.
LinkOut - more resources
Full Text Sources
Other Literature Sources
