Routine use of immunosuppressants is associated with mortality in hospitalised patients with COVID-19
- PMID: 33680426
- PMCID: PMC7897811
- DOI: 10.1177/2042098620985690
Routine use of immunosuppressants is associated with mortality in hospitalised patients with COVID-19
Abstract
Background: Whilst there is literature on the impact of SARS viruses in the severely immunosuppressed, less is known about the link between routine immunosuppressant use and outcome in COVID-19. Consequently, guidelines on their use vary depending on specific patient populations.
Methods: The study population was drawn from the COPE Study (COVID-19 in Older People), a multicentre observational cohort study, across the UK and Italy. Data were collected between 27 February and 28 April 2020 by trained data-collectors and included all unselected consecutive admissions with COVID-19. Load (name/number of medications) and dosage of immunosuppressant were collected along with other covariate data. Primary outcome was time-to-mortality from the date of admission (or) date of diagnosis, if diagnosis was five or more days after admission. Secondary outcomes were Day-14 mortality and time-to-discharge. Data were analysed with mixed-effects, Cox proportional hazards and logistic regression models using non-users of immunosuppressants as the reference group.
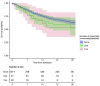
Results: In total 1184 patients were eligible for inclusion. The median (IQR) age was 74 (62-83), 676 (57%) were male, and 299 (25.3%) died in hospital (total person follow-up 15,540 days). Most patients exhibited at least one comorbidity, and 113 (~10%) were on immunosuppressants. Any immunosuppressant use was associated with increased mortality: aHR 1.87, 95% CI: 1.30, 2.69 (time to mortality) and aOR 1.71, 95% CI: 1.01-2.88 (14-day mortality). There also appeared to be a dose-response relationship.
Conclusion: Despite possible indication bias, until further evidence emerges we recommend adhering to public health measures, a low threshold to seek medical advice and close monitoring of symptoms in those who take immunosuppressants routinely regardless of their indication. However, it should be noted that the inability to control for the underlying condition requiring immunosuppressants is a major limitation, and hence caution should be exercised in interpretation of the results.
Plain language summary: Regular Use of Immune Suppressing Drugs is Associated with Increased Risk of Death in Hospitalised Patients with COVID-19 Background: We do not have much information on how the COVID-19 virus affects patients who use immunosuppressants, drugs which inhibit or reduce the activity of the immune system. There are various conflicting views on whether immune-suppressing drugs are beneficial or detrimental in patients with the disease. Methods: This study collected data from 10 hospitals in the UK and one in Italy between February and April 2020 in order to identify any association between the regular use of immunosuppressant medicines and survival in patients who were admitted to hospital with COVID-19. Results: 1184 patients were included in the study, and 10% of them were using immunosuppressants. Any immunosuppressant use was associated with increased risk of death, and the risk appeared to increase if the dose of the medicine was higher. Conclusion: We therefore recommend that patients who take immunosuppressant medicines routinely should carefully adhere to social distancing measures, and seek medical attention early during the COVID-19 pandemic.
Keywords: Coronavirus; Covid-19; immunosuppressants; immunosuppression.
© The Author(s), 2021.
Conflict of interest statement
Conflict of interest statement: The authors declare that there is no conflict of interest.
Figures
Similar articles
-
Virtualized clinical studies to assess the natural history and impact of gut microbiome modulation in non-hospitalized patients with mild to moderate COVID-19 a randomized, open-label, prospective study with a parallel group study evaluating the physiologic effects of KB109 on gut microbiota structure and function: a structured summary of a study protocol for a randomized controlled study.Trials. 2021 Apr 2;22(1):245. doi: 10.1186/s13063-021-05157-0. Trials. 2021. PMID: 33810796 Free PMC article.
-
Risk Factors Associated With Mortality Among Patients With COVID-19 in Intensive Care Units in Lombardy, Italy.JAMA Intern Med. 2020 Oct 1;180(10):1345-1355. doi: 10.1001/jamainternmed.2020.3539. JAMA Intern Med. 2020. PMID: 32667669 Free PMC article.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Guidelines for Cervical Cancer Screening in Immunosuppressed Women Without HIV Infection.J Low Genit Tract Dis. 2019 Apr;23(2):87-101. doi: 10.1097/LGT.0000000000000468. J Low Genit Tract Dis. 2019. PMID: 30907775
-
Systematic review of immunosuppressant guidelines in the COVID-19 pandemic.Ther Adv Drug Saf. 2021 Feb 10;12:2042098620985687. doi: 10.1177/2042098620985687. eCollection 2021. Ther Adv Drug Saf. 2021. PMID: 33628418 Free PMC article. Review.
Cited by
-
Could immunotherapy and regulatory T cells be used therapeutically to slow the progression of Alzheimer's disease?Brain Commun. 2025 Feb 25;7(2):fcaf092. doi: 10.1093/braincomms/fcaf092. eCollection 2025. Brain Commun. 2025. PMID: 40078868 Free PMC article. Review.
-
Using artificial intelligence to expedite and enhance plain language summary abstract writing of scientific content.JAMIA Open. 2025 Apr 3;8(2):ooaf023. doi: 10.1093/jamiaopen/ooaf023. eCollection 2025 Apr. JAMIA Open. 2025. PMID: 40183004 Free PMC article.
-
One-Year Report of COVID-19 Impact on Geriatric Patients: a Bio-Psycho-Social Approach.Can Geriatr J. 2022 Jun 1;25(2):212-221. doi: 10.5770/cgj.25.553. eCollection 2022 Jun. Can Geriatr J. 2022. PMID: 35747408 Free PMC article. Review.
-
Disparities in COVID-19 mortality amongst the immunosuppressed: A systematic review and meta-analysis for enhanced disease surveillance.J Infect. 2024 Mar;88(3):106110. doi: 10.1016/j.jinf.2024.01.009. Epub 2024 Jan 30. J Infect. 2024. PMID: 38302061 Free PMC article.
-
Evaluation of coronavirus diseases (COVID-19) in terms of epidemiological and clinical features, comorbidities, diagnostic methods, treatment, and mortality.J Educ Health Promot. 2022 Jul 29;11:236. doi: 10.4103/jehp.jehp_1328_21. eCollection 2022. J Educ Health Promot. 2022. PMID: 36177413 Free PMC article.
References
-
- University of Oxford. RECOVERY. 2020. Nuffield department of population health, https://www.recoverytrial.net/files/recovery_dexamethasone_statement_160... (accessed 22 June 2020).
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous