RGB-color forward-viewing spectrally encoded endoscope using three orders of diffraction
- PMID: 33680558
- PMCID: PMC7901315
- DOI: 10.1364/BOE.415852
RGB-color forward-viewing spectrally encoded endoscope using three orders of diffraction
Abstract
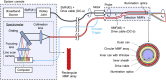
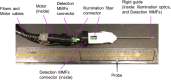
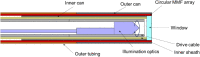
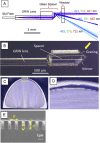
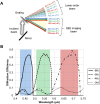
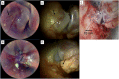
Spectrally encoded endoscopy (SEE) is an ultra-miniature endoscopy technology that encodes each spatial location on the sample with a different wavelength. One challenge in SEE is achieving color imaging with a small probe. We present a novel SEE probe that is capable of conducting real-time RGB imaging using three diffraction orders (6th order diffraction of the blue spectrum, 5th of green, and 4th of red). The probe was comprised of rotating 0.5 mm-diameter illumination optics inside a static, 1.2 mm-diameter flexible sheath with a rigid distal length of 5 mm containing detection fibers. A color chart, resolution target, and swine tissue were imaged. The device achieved 44k/59k/23k effective pixels per R/G/B channels over a 58° angular field and differentiated a wide gamut of colors.
© 2021 Optical Society of America under the terms of the OSA Open Access Publishing Agreement.
Conflict of interest statement
This research was sponsored by Canon U.S.A., Inc. MI, TW, ATM, AA, XY, JHH, ST(Takeuchi): Canon U.S.A., Inc. (E, P). AY, ST(Tatsumi), KI: Canon Inc. (E, P). JR, AZ, GJT: Canon U.S.A., Inc. (F, P, R).
Figures



Similar articles
-
High-Resolution, Wide-Field, Forward-Viewing Spectrally Encoded Endoscope.Lasers Surg Med. 2019 Nov;51(9):808-814. doi: 10.1002/lsm.23102. Epub 2019 May 26. Lasers Surg Med. 2019. PMID: 31129921
-
Single-beam spectrally encoded color imaging.Opt Lett. 2018 May 15;43(10):2229-2232. doi: 10.1364/OL.43.002229. Opt Lett. 2018. PMID: 29762558
-
Spectral imaging using forward-viewing spectrally encoded endoscopy.Biomed Opt Express. 2016 Jan 8;7(2):392-8. doi: 10.1364/BOE.7.000392. eCollection 2016 Feb 1. Biomed Opt Express. 2016. PMID: 26977348 Free PMC article.
-
The simple perfection of quantum correlation in human vision.Prog Neurobiol. 2006 Jan;78(1):38-60. doi: 10.1016/j.pneurobio.2005.11.006. Epub 2005 Dec 27. Prog Neurobiol. 2006. PMID: 16377059 Review.
-
Full-Color Realization of Micro-LED Displays.Nanomaterials (Basel). 2020 Dec 10;10(12):2482. doi: 10.3390/nano10122482. Nanomaterials (Basel). 2020. PMID: 33322057 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
