Biocompatibility and bioactive potential of the NeoMTA Plus endodontic bioceramic-based sealer
- PMID: 33680893
- PMCID: PMC7906839
- DOI: 10.5395/rde.2021.46.e4
Biocompatibility and bioactive potential of the NeoMTA Plus endodontic bioceramic-based sealer
Abstract
Objectives: This study evaluated the biocompatibility and bioactive potential of NeoMTA Plus mixed as a root canal sealer in comparison with MTA Fillapex.
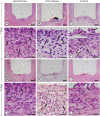
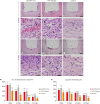
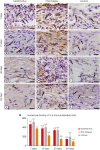
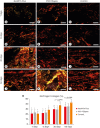
Materials and methods: Polyethylene tubes filled with NeoMTA Plus (n = 20), MTA Fillapex (n = 20), or nothing (control group, CG; n = 20) were inserted into the connective tissue in the dorsal subcutaneous layer of rats. After 7, 15, 30 and 60 days, the specimens were processed for paraffin embedding. The capsule thickness, collagen content, and number of inflammatory cells (ICs) and interleukin-6 (IL-6) immunolabeled cells were measured. von Kossa-positive structures were evaluated and unstained sections were analyzed under polarized light. Two-way analysis of variance was performed, followed by the post hoc Tukey test (p ≤ 0.05).
Results: At 7 days, the capsules around NeoMTA Plus and MTA Fillapex had more ICs and IL-6-immunostained cells than the CG. However, at 60 days, there was no significant difference in the IC number between NeoMTA Plus and the CG (p = 0.1137) or the MTA Fillapex group (p = 0.4062), although a greater number of IL-6-immunostained cells was observed in the MTA Fillapex group (p = 0.0353). From 7 to 60 days, the capsule thickness of the NeoMTA Plus and MTA Fillapex specimens significantly decreased, concomitantly with an increase in the collagen content. The capsules around root canal sealers showed positivity to the von Kossa stain and birefringent structures.
Conclusions: The NeoMTA Plus root canal sealer is biocompatible and exhibits bioactive potential.
Keywords: Bioactive potential; Biocompatibility; Immunohistochemistry; Inflammatory reaction; Interleukin-6.
Copyright © 2021. The Korean Academy of Conservative Dentistry.
Conflict of interest statement
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
Figures

References
-
- Tomás-Catalá CJ, Collado-González M, García-Bernal D, Oñate-Sánchez RE, Forner L, Llena C, Lozano A, Moraleda JM, Rodríguez-Lozano FJ. Biocompatibility of new pulp-capping materials NeoMTA Plus, MTA Repair HP, and biodentine on human dental pulp stem cells. J Endod. 2018;44:126–132. - PubMed
-
- Cintra LTA, Benetti F, de Azevedo Queiroz ÍO, de Araújo Lopes JM, Penha de Oliveira SH, Sivieri Araújo G, Gomes-Filho JE. Cytotoxicity, biocompatibility, and biomineralization of the new high-plasticity MTA Material. J Endod. 2017;43:774–778. - PubMed
-
- Mondelli JAS, Hoshino RA, Weckwerth PH, Cerri PS, Leonardo RT, Guerreiro-Tanomaru JM, Tanomaru-Filho M, da Silva GF. Biocompatibility of mineral trioxide aggregate flow and biodentine. Int Endod J. 2019;52:193–200. - PubMed
-
- Parirokh M, Torabinejad M, Dummer PMH. Mineral trioxide aggregate and other bioactive endodontic cements: an updated overview - Part I: Vital pulp therapy. Int Endod J. 2018;51:177–205. - PubMed
-
- Siboni F, Taddei P, Prati C, Gandolfi MG. Properties of NeoMTA Plus and MTA Plus cements for endodontics. Int Endod J. 2017;50(Supplement 2):e83–e94. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

