Pentoxifylline Sensitizes Cisplatin-Resistant Human Cervical Cancer Cells to Cisplatin Treatment: Involvement of Mitochondrial and NF-Kappa B Pathways
- PMID: 33680921
- PMCID: PMC7931705
- DOI: 10.3389/fonc.2020.592706
Pentoxifylline Sensitizes Cisplatin-Resistant Human Cervical Cancer Cells to Cisplatin Treatment: Involvement of Mitochondrial and NF-Kappa B Pathways
Abstract
Background: Cervical cancer continues to be a major public health problem worldwide, and Cisplatin is used as first-line chemotherapy for this cancer; however, malignant cells exposed to CISplatin (CIS) become insensitive to the effects of this drug. PenToXifylline (PTX) is a xanthine that sensitizes several types of tumor cells to apoptosis induced by antitumor drugs, such as Adriamycin, Carboplatin, and CIS. The effects of PTX on tumor cells have been related to the disruption of the NF-κB pathway, thus preventing the activation of cell survival mechanisms such as the expression of anti-apoptotic genes, the secretion of proinflammatory interleukins, and growth factors.
Objective: In this work, we studied the antitumor proprieties of PTX in human SiHa cervical carcinoma cells resistant to CIS.
Materials and methods: SiHa and HeLa cervical cancer cells and their CIS-resistant derived cell lines (SiHaCIS-R and HeLaCIS-R, respectively) were used as in-vitro models. We studied the effects of PTX alone or in combination with CIS on cell viability, apoptosis, caspase-3, caspase-8, and caspase-9 activity, cleaved PARP-1, anti-apoptotic protein (Bcl-2 and Bcl-xL) levels, p65 phosphorylation, cadmium chloride (CdCl2) sensitivity, Platinum (Pt) accumulation, and glutathione (GSH) levels, as well as on the gene expression of GSH and drug transporters (influx and efflux).
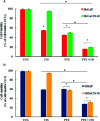
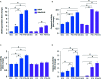
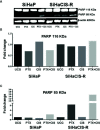
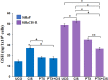
Results: PTX sensitized SiHaCIS-R cells to the effects of CIS by inducing apoptosis, caspase activation, and PARP-1 cleavage. PTX treatment also decreased p65 phosphorylation, increased Pt levels, depleted GSH, and downregulated the expression of the ATP7A, ATP7B, GSR, and MGST1 genes.
Conclusion: PTX reverses the acquired phenotype of CIS resistance close to the sensitivity of parental SiHa cells.
Keywords: NF-KappaB; cervical cancer cells; chemoresistance; cisplatin; pentoxifylline.
Copyright © 2020 Bravo-Cuellar, Ortiz-Lazareno, Sierra-Díaz, Solorzano-Ibarra, Méndez-Clemente, Aguilar-Lemarroy, Jave-Suárez, Ruiz Velazco-Niño and Hernández-Flores.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Chemotherapy with a molecular rational basis, pentoxifylline as a promising antitumor drug.Ann Med Surg (Lond). 2025 Feb 28;87(3):1506-1528. doi: 10.1097/MS9.0000000000003043. eCollection 2025 Mar. Ann Med Surg (Lond). 2025. PMID: 40213176 Free PMC article. Review.
-
Pentoxifylline sensitizes human cervical tumor cells to cisplatin-induced apoptosis by suppressing NF-kappa B and decreased cell senescence.BMC Cancer. 2011 Nov 10;11:483. doi: 10.1186/1471-2407-11-483. BMC Cancer. 2011. PMID: 22074157 Free PMC article.
-
Sensitization of cervix cancer cells to Adriamycin by Pentoxifylline induces an increase in apoptosis and decrease senescence.Mol Cancer. 2010 May 19;9:114. doi: 10.1186/1476-4598-9-114. Mol Cancer. 2010. PMID: 20482878 Free PMC article.
-
Pentoxifylline Enhances the Apoptotic Effect of Carboplatin in Y79 Retinoblastoma Cells.In Vivo. 2019 Mar-Apr;33(2):401-412. doi: 10.21873/invivo.11487. In Vivo. 2019. PMID: 30804118 Free PMC article.
-
Pentoxifylline and the proteasome inhibitor MG132 induce apoptosis in human leukemia U937 cells through a decrease in the expression of Bcl-2 and Bcl-XL and phosphorylation of p65.J Biomed Sci. 2013 Feb 28;20(1):13. doi: 10.1186/1423-0127-20-13. J Biomed Sci. 2013. PMID: 23445492 Free PMC article.
Cited by
-
Improvement of Docetaxel Efficacy through Simultaneous Blockade of Transcription Factors NF-κB and STAT-3 Using Pentoxifylline and Stattic in Prostate Cancer Cells.Curr Issues Mol Biol. 2024 Sep 14;46(9):10140-10159. doi: 10.3390/cimb46090605. Curr Issues Mol Biol. 2024. PMID: 39329957 Free PMC article.
-
Microsomal glutathione transferase 1 in cancer and the regulation of ferroptosis.Adv Cancer Res. 2023;160:107-132. doi: 10.1016/bs.acr.2023.05.001. Epub 2023 Jul 21. Adv Cancer Res. 2023. PMID: 37704286 Free PMC article. Review.
-
Cisplatin-Based Combination Therapy for Enhanced Cancer Treatment.Curr Drug Targets. 2024;25(7):473-491. doi: 10.2174/0113894501294182240401060343. Curr Drug Targets. 2024. PMID: 38591210 Review.
-
TNF-α induces up-regulation of MicroRNA-27a via the P38 signalling pathway, which inhibits intervertebral disc degeneration by targeting FSTL1.J Cell Mol Med. 2021 Aug;25(15):7146-7156. doi: 10.1111/jcmm.16745. Epub 2021 Jun 30. J Cell Mol Med. 2021. PMID: 34190406 Free PMC article.
-
Chemotherapy with a molecular rational basis, pentoxifylline as a promising antitumor drug.Ann Med Surg (Lond). 2025 Feb 28;87(3):1506-1528. doi: 10.1097/MS9.0000000000003043. eCollection 2025 Mar. Ann Med Surg (Lond). 2025. PMID: 40213176 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

