Evaluation of 18F-DCFPyL PSMA PET/CT for Prostate Cancer: A Meta-Analysis
- PMID: 33680924
- PMCID: PMC7925846
- DOI: 10.3389/fonc.2020.597422
Evaluation of 18F-DCFPyL PSMA PET/CT for Prostate Cancer: A Meta-Analysis
Abstract
Background: To systematically review the clinical value of 18F-DCFPyL prostate-specific membrane antigen positron emission tomography/computed tomography (PSMA PET/CT) in the diagnosis of prostate cancer (PCa).
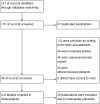
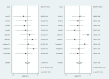
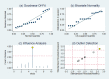
Methods: Literature concerning 18F-DCFPyL PSMA PET/CT in the diagnosis of prostate cancer published from 2015 to 2020 was electronically searched in the databases including PubMed and Embase. Statistical analysis was carried out with STATA 15 software, and the quality of included studies was tested with quality assessment of diagnostic accuracy studies (QUADAS) items. The heterogeneity of the included data was tested.
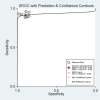
Results: In total, nine pieces of literature involving 426 patients met the inclusion criteria. The heterogeneity of the study group was not obvious. The SEN, SPE, LR+, LR-, DOR as well as AUC of 18F-DCFPyL PSMA PET/CT diagnosis of prostate cancer were 0.91, 0.90, 8.9, 0.10, 93, and 0.93. The pooled DR of 18F-DCFPyL labeled PSMA PET/CT in PCa was 92%. The pooled DR was 89% for PSA≥0.5 ng/ml and 49% for PSA < 0.5ng/ml.
Conclusion: 18F-DCFPyL PSMA PET/CT had good sensitivity and specificity for the diagnosis of prostate cancer. The DR of 18F-DCFPyL PSMA PET/CT was correlated with PSA value. Further large-sample, high-quality studies were needed.
Keywords: 18F-DCFPyL PSMA PET/CT; diagnosis; imaging; meta-analysis; prostate cancer.
Copyright © 2021 Pan, Wang, Wang, Nikzad, Kong, Jian, Zhang, Lu, Xu, Wang and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Performance of 18F-DCFPyL PET/CT Imaging in Early Detection of Biochemically Recurrent Prostate Cancer: A Systematic Review and Meta-Analysis.Front Oncol. 2021 Apr 26;11:649171. doi: 10.3389/fonc.2021.649171. eCollection 2021. Front Oncol. 2021. PMID: 33981607 Free PMC article.
-
Diagnostic performance of 18F-labeled PSMA PET/CT in patients with biochemical recurrence of prostate cancer: a systematic review and meta-analysis.Acta Radiol. 2023 Oct;64(10):2791-2801. doi: 10.1177/02841851231184210. Epub 2023 Aug 6. Acta Radiol. 2023. PMID: 37545168
-
The performance of 18F-PSMA PET/CT in the detection of prostate cancer: a systematic review and meta-analysis.Asian J Androl. 2022 Jul-Aug;24(4):373-379. doi: 10.4103/aja202162. Asian J Androl. 2022. PMID: 34747721 Free PMC article.
-
The diagnostic performance of 18F-DCFPyL PET in patients with suspected prostate cancer: A systemic review and meta-analysis.Front Oncol. 2023 Mar 7;13:1145759. doi: 10.3389/fonc.2023.1145759. eCollection 2023. Front Oncol. 2023. PMID: 36959787 Free PMC article.
-
Diagnostic performance of 18F‑DCFPyL PET vs. 68Ga‑PSMA PET/CT in patients with suspected prostate cancer: A systemic review and meta‑analysis.Oncol Lett. 2024 Feb 29;27(4):188. doi: 10.3892/ol.2024.14321. eCollection 2024 Apr. Oncol Lett. 2024. PMID: 38486944 Free PMC article.
Cited by
-
PSMA PET in Imaging Prostate Cancer.Front Oncol. 2022 Jan 28;12:831429. doi: 10.3389/fonc.2022.831429. eCollection 2022. Front Oncol. 2022. PMID: 35155262 Free PMC article. Review.
-
A Preliminary Study of PSMA Fluorescent Probe for Targeted Fluorescence Imaging of Prostate Cancer.Molecules. 2022 Apr 24;27(9):2736. doi: 10.3390/molecules27092736. Molecules. 2022. PMID: 35566085 Free PMC article.
-
Affinity probes based on small-molecule inhibitors for tumor imaging.Front Oncol. 2022 Oct 27;12:1028493. doi: 10.3389/fonc.2022.1028493. eCollection 2022. Front Oncol. 2022. PMID: 36387103 Free PMC article. Review.
-
PSMA-Targeting Imaging and Theranostic Agents-Current Status and Future Perspective.Int J Mol Sci. 2022 Jan 21;23(3):1158. doi: 10.3390/ijms23031158. Int J Mol Sci. 2022. PMID: 35163083 Free PMC article. Review.
-
18F-DCFPyL (PSMA) PET as a radiotherapy response assessment tool in metastatic prostate cancer.Clin Transl Radiat Oncol. 2023 Jan 18;39:100583. doi: 10.1016/j.ctro.2023.100583. eCollection 2023 Mar. Clin Transl Radiat Oncol. 2023. PMID: 36713978 Free PMC article.
References
-
- Daniyal M, Siddiqui ZA, Akram M, Asif HM, Sultana S, Khan A. Epidemiology, etiology, diagnosis and treatment of prostate cancer. Asian Pac J Cancer Prev (2014) 15(22):9575–8. - PubMed
-
- Kopka K, Benesova M, Barinka C, et al. . Glu-ureido-based inhibtros of prostate-specific membrane antigen: lessons learned during the development of a novel class of low-molecular-weight theranostic radiotracers. J Nucl Med (2017) 58(Suppl 2):17S–26S. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin (2018) 68(1):7–30. - PubMed
-
- Tao Z-Q, ShiI A-M, Wang K-X, Zhang W-D. Epidemiology of prostate cancer: Current status. Eur Rev Med Pharmacol Sci (2015) 19(5):805–12. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

