Radiomics Signature Facilitates Organ-Saving Strategy in Patients With Esophageal Squamous Cell Cancer Receiving Neoadjuvant Chemoradiotherapy
- PMID: 33680935
- PMCID: PMC7933499
- DOI: 10.3389/fonc.2020.615167
Radiomics Signature Facilitates Organ-Saving Strategy in Patients With Esophageal Squamous Cell Cancer Receiving Neoadjuvant Chemoradiotherapy
Abstract
After neoadjuvant chemoradiotherapy (NCRT) in locally advanced esophageal squamous cell cancer (ESCC), roughly 40% of the patients may achieve pathologic complete response (pCR). Those patients may benefit from organ-saving strategy if the probability of pCR could be correctly identified before esophagectomy. A reliable approach to predict pathological response allows future studies to investigate individualized treatment plans.
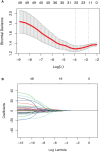
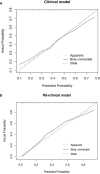
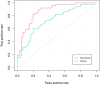
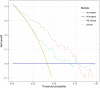
Method: All eligible patients treated in our center from June 2012 to June 2019 were retrospectively collected. Radiomics features extracted from pre-/post-NCRT CT images were selected by univariate logistic and LASSO regression. A radiomics signature (RS) developed with selected features was combined with clinical variables to construct RS+clinical model with multivariate logistic regression, which was internally validated by bootstrapping. Performance and clinical usefulness of RS+clinical model were assessed by receiver operating characteristic (ROC) curves and decision curve analysis, respectively.
Results: Among the 121 eligible patients, 51 achieved pCR (42.1%) after NCRT. Eighteen radiomics features were selected and incorporated into RS. The RS+clinical model has improved prediction performance for pCR compared with the clinical model (corrected area under the ROC curve, 0.84 vs. 0.70). At the 60% probability threshold cutoff (i.e., the patient would opt for observation if his probability of pCR was >60%), net 13% surgeries could be avoided by RS+clinical model, equivalent to implementing organ-saving strategy in 31.37% of the 51 true-pCR cases.
Conclusion: The model built with CT radiomics features and clinical variables shows the potential of predicting pCR after NCRT; it provides significant clinical benefit in identifying qualified patients to receive individualized organ-saving treatment plans.
Keywords: esophageal cancer; neoadjuvant chemoradiation; organ-saving treatment; radiomics; response prediction.
Copyright © 2021 Li, Liu, Li, Cai, Li, Ye, Teng, Fu and Yu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Shapiro J, van Lanschot JJB, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, Wijnhoven Bas PL, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol (2015) 16(9):1090–8. 10.1016/S1470-2045(15)00040-6 - DOI - PubMed
-
- van Rossum PSN, Fried DV, Zhang L, Hofstetter WL, van Vulpen M, Meijer GJ, et al. The Incremental Value of Subjective and Quantitative Assessment of 18F-FDG PET for the Prediction of Pathologic Complete Response to Preoperative Chemoradiotherapy in Esophageal Cancer. J Nucl Med (2016) 57(5):691–700. 10.2967/jnumed.115.163766 - DOI - PubMed
-
- Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial. J Clin Oncol (2018) 36(27):2796–803. 10.1200/JCO.2018.79.1483 - DOI - PMC - PubMed
-
- Klevebro F, Alexandersson von Döbeln G, Wang N, Johnsen G, Jacobsen A-B, Friesland S, et al. A randomized clinical trial of neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the oesophagus or gastro-oesophageal junction. Ann Oncol (2016) 27(4):660–7. 10.1093/annonc/mdw010 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

