Synergism of Streptococcus mutans and Candida albicans Reinforces Biofilm Maturation and Acidogenicity in Saliva: An In Vitro Study
- PMID: 33680985
- PMCID: PMC7933670
- DOI: 10.3389/fcimb.2020.623980
Synergism of Streptococcus mutans and Candida albicans Reinforces Biofilm Maturation and Acidogenicity in Saliva: An In Vitro Study
Abstract
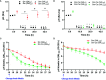
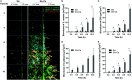
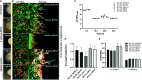
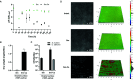
Early childhood caries, a virulent-form of dental caries, is painful, difficult, and costly to treat that has been associated with high levels of Streptococcus mutans (Sm) and Candida albicans (Ca) in plaque-biofilms on teeth. These microorganisms appear to develop a symbiotic cross-kingdom interaction that amplifies the virulence of plaque-biofilms. Although biofilm studies reveal synergistic bacterial-fungal association, how these organisms modulate cross-kingdom biofilm formation and enhance its virulence in the presence of saliva remain largely unknown. Here, we compared the properties of Sm and Sm-Ca biofilms cultured in saliva by examining the biofilm structural organization and capability to sustain an acidic pH environment conducive to enamel demineralization. Intriguingly, Sm-Ca biofilm is rapidly matured and maintained acidic pH-values (~4.3), while Sm biofilm development was retarded and failed to create an acidic environment when cultured in saliva. In turn, the human enamel slab surface was severely demineralized by Sm-Ca biofilms, while there was minimal damage to the enamel surface by Sm biofilm. Interestingly, Sm-Ca biofilms exhibited an acidic environment regardless of their hyphal formation ability. Our data reveal the critical role of symbiotic interaction between S. mutans and C. albicans in human saliva in the context of pathogenesis of dental caries, which may explain how the cross-kingdom interaction contributes to enhanced virulence of plaque-biofilm in the oral cavity.
Keywords: Candida albicans; Streptococcus mutans; acidogenicity; cross-kingdom biofilm; enamel demineralization; human saliva.
Copyright © 2021 Kim, Liu, Dhall, Bawazir, Koo and Hwang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

