Human Zona Pellucida Glycoproteins: Binding Characteristics With Human Spermatozoa and Induction of Acrosome Reaction
- PMID: 33681199
- PMCID: PMC7928326
- DOI: 10.3389/fcell.2021.619868
Human Zona Pellucida Glycoproteins: Binding Characteristics With Human Spermatozoa and Induction of Acrosome Reaction
Abstract
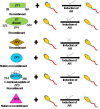
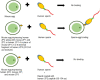
Human zona pellucida (ZP) matrix is composed of four glycoproteins designated as ZP glycoprotein -1 (ZP1), -2 (ZP2), -3 (ZP3), and -4 (ZP4). Mutations in the genes encoding human ZP glycoproteins are one of the causative factors leading to abnormal ZP matrix and infertility in women. Relevance of the human ZP glycoproteins in 'sperm-oocyte' binding has been delineated by using either transgenic animal models expressing human zona proteins or purified native/recombinant human zona proteins. Studies based on the purified native/recombinant human zona proteins revealed that ZP1, ZP3, and ZP4 primarily bind to the capacitated acrosome-intact human spermatozoa whereas ZP2 binds to acrosome-reacted spermatozoa. On the contrary, human spermatozoa binds to the eggs obtained from transgenic mouse lines expressing human ZP2 but not to those expressing human ZP1, ZP3, and ZP4 suggesting that ZP2 has an important role in human 'sperm-oocyte' binding. Further studies using transgenic mouse lines showed that the N-terminus of human ZP2 mediate the taxon-specific human sperm-oocyte binding. Both glycans and protein-protein interactions have a role in human gamete interaction. Further studies have revealed that the purified native/recombinant human ZP1, ZP3, and ZP4 are competent to induce acrosome reaction. Human sperm binds to the mouse transgenic eggs expressing human ZP1-4 instead of mouse ZP1-3 proteins, penetrated the ZP matrix and accumulated in the perivitelline space, which were acrosome-reacted suggesting that human ZP2 in transgenic mouse model also induce acrosome reaction. In humans N-linked glycosylation of zona proteins have been shown to play an important role in induction of the acrosome reaction. Hence in humans, based on studies using transgenic mouse model as well as purified native/recombinant zona proteins, it is likely that more than one zona protein is involved in the 'sperm-oocyte' binding and induction of the acrosome reaction.
Keywords: ZP glycoproteins-mediated acrosome reaction; fertilization; human zona pellucida glycoproteins; mutations in genes encoding human zona glycoproteins; zona proteins binding to sperm.
Copyright © 2021 Gupta.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
The Human Egg's Zona Pellucida.Curr Top Dev Biol. 2018;130:379-411. doi: 10.1016/bs.ctdb.2018.01.001. Epub 2018 Mar 6. Curr Top Dev Biol. 2018. PMID: 29853184 Review.
-
Mammalian zona pellucida glycoproteins: structure and function during fertilization.Cell Tissue Res. 2012 Sep;349(3):665-78. doi: 10.1007/s00441-011-1319-y. Cell Tissue Res. 2012. PMID: 22298023 Review.
-
Role of zona pellucida glycoproteins during fertilization in humans.J Reprod Immunol. 2015 Apr;108:90-7. doi: 10.1016/j.jri.2014.08.006. Epub 2014 Oct 5. J Reprod Immunol. 2015. PMID: 25445843 Review.
-
Human zona pellucida glycoproteins: functional relevance during fertilization.J Reprod Immunol. 2009 Dec;83(1-2):50-5. doi: 10.1016/j.jri.2009.07.008. Epub 2009 Oct 21. J Reprod Immunol. 2009. PMID: 19850354 Review.
-
Acrosome reaction: relevance of zona pellucida glycoproteins.Asian J Androl. 2011 Jan;13(1):97-105. doi: 10.1038/aja.2010.72. Epub 2010 Nov 1. Asian J Androl. 2011. PMID: 21042299 Free PMC article. Review.
Cited by
-
Gene mutations associated with fertilization failure after in vitro fertilization/intracytoplasmic sperm injection.Front Endocrinol (Lausanne). 2022 Dec 16;13:1086883. doi: 10.3389/fendo.2022.1086883. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36589837 Free PMC article. Review.
-
NHR-23 activity is necessary for C. elegans developmental progression and apical extracellular matrix structure and function.Development. 2023 May 15;150(10):dev201085. doi: 10.1242/dev.201085. Epub 2023 May 22. Development. 2023. PMID: 37129010 Free PMC article.
-
Advances in Molecular Biology and Immunology of Spermatozoa and Fertilization in Domestic Animals: Implications for Infertility and Assisted Reproduction.Curr Mol Med. 2025;25(2):167-186. doi: 10.2174/0115665240306965240802075331. Curr Mol Med. 2025. PMID: 39572916 Review.
-
Discordant effects of maternal age on the human MII oocyte transcriptome.Mol Hum Reprod. 2025 Jul 3;31(3):gaaf038. doi: 10.1093/molehr/gaaf038. Mol Hum Reprod. 2025. PMID: 40729310 Free PMC article.
-
Novel mutations in ZP2 and ZP3 cause female infertility in three patients.J Assist Reprod Genet. 2022 May;39(5):1205-1215. doi: 10.1007/s10815-022-02466-4. Epub 2022 Apr 3. J Assist Reprod Genet. 2022. PMID: 35366744 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

