Brain Ultrastructure: Putting the Pieces Together
- PMID: 33681208
- PMCID: PMC7930431
- DOI: 10.3389/fcell.2021.629503
Brain Ultrastructure: Putting the Pieces Together
Abstract
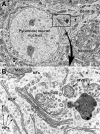
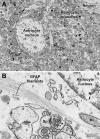
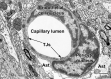
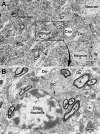
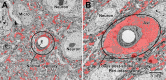
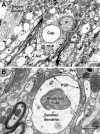
Unraveling the fine structure of the brain is important to provide a better understanding of its normal and abnormal functioning. Application of high-resolution electron microscopic techniques gives us an unprecedented opportunity to discern details of the brain parenchyma at nanoscale resolution, although identifying different cell types and their unique features in two-dimensional, or three-dimensional images, remains a challenge even to experts in the field. This article provides insights into how to identify the different cell types in the central nervous system, based on nuclear and cytoplasmic features, amongst other unique characteristics. From the basic distinction between neurons and their supporting cells, the glia, to differences in their subcellular compartments, organelles and their interactions, ultrastructural analyses can provide unique insights into the changes in brain function during aging and disease conditions, such as stroke, neurodegeneration, infection and trauma. Brain parenchyma is composed of a dense mixture of neuronal and glial cell bodies, together with their intertwined processes. Intracellular components that vary between cells, and can become altered with aging or disease, relate to the cytoplasmic and nucleoplasmic density, nuclear heterochromatin pattern, mitochondria, endoplasmic reticulum and Golgi complex, lysosomes, neurosecretory vesicles, and cytoskeletal elements (actin, intermediate filaments, and microtubules). Applying immunolabeling techniques to visualize membrane-bound or intracellular proteins in neurons and glial cells gives an even better appreciation of the subtle differences unique to these cells across contexts of health and disease. Together, our observations reveal how simple ultrastructural features can be used to identify specific changes in cell types, their health status, and functional relationships in the brain.
Keywords: aging; brain; disease; electron microscopy; glial cells; health; neurons; organelles.
Copyright © 2021 Nahirney and Tremblay.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

