Tendon Stem/Progenitor Cell Subpopulations and Their Implications in Tendon Biology
- PMID: 33681210
- PMCID: PMC7930382
- DOI: 10.3389/fcell.2021.631272
Tendon Stem/Progenitor Cell Subpopulations and Their Implications in Tendon Biology
Abstract
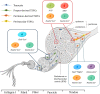
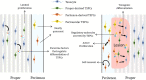
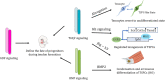
Tendon harbors a cell population that possesses stem cell characteristics such as clonogenicity, multipotency and self-renewal capacity, commonly referred to as tendon stem/progenitor cells (TSPCs). Various techniques have been employed to study how TSPCs are implicated in tendon development, homeostasis and healing. Recent advances in single-cell analysis have enabled much progress in identifying and characterizing distinct subpopulations of TSPCs, which provides a more comprehensive view of TSPCs function in tendon biology. Understanding the mechanisms of physiological and pathological processes regulated by TSPCs, especially a particular subpopulation, would greatly benefit treatment of diseased tendons. Here, we summarize the current scientific literature on the various subpopulations of TSPCs, and discuss how TSPCs can contribute to tissue homeostasis and pathogenesis, as well as examine the key modulatory signaling pathways that determine stem/progenitor cell state. A better understanding of the roles that TSPCs play in tendon biology may facilitate the development of novel treatment strategies for tendon diseases.
Keywords: TGFβ; healing; niche; subpopulation; tendon stem/progenitor cells.
Copyright © 2021 Huang, Yin, Xu, Fei, Heng, Jiang, Chen and Shen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
The Functions and Mechanisms of Tendon Stem/Progenitor Cells in Tendon Healing.Stem Cells Int. 2023 Sep 12;2023:1258024. doi: 10.1155/2023/1258024. eCollection 2023. Stem Cells Int. 2023. PMID: 37731626 Free PMC article. Review.
-
Single-cell analysis reveals a nestin+ tendon stem/progenitor cell population with strong tenogenic potentiality.Sci Adv. 2016 Nov 18;2(11):e1600874. doi: 10.1126/sciadv.1600874. eCollection 2016 Nov. Sci Adv. 2016. PMID: 28138519 Free PMC article.
-
Targeting Senescent Tendon Stem/Progenitor Cells to Prevent or Treat Age-Related Tendon Disorders.Stem Cell Rev Rep. 2023 Apr;19(3):680-693. doi: 10.1007/s12015-022-10488-9. Epub 2022 Dec 15. Stem Cell Rev Rep. 2023. PMID: 36520409 Review.
-
Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche.Nat Med. 2007 Oct;13(10):1219-27. doi: 10.1038/nm1630. Epub 2007 Sep 9. Nat Med. 2007. PMID: 17828274
-
Downregulation of FOXP1 correlates with tendon stem/progenitor cells aging.Biochem Biophys Res Commun. 2018 Sep 26;504(1):96-102. doi: 10.1016/j.bbrc.2018.08.136. Epub 2018 Aug 29. Biochem Biophys Res Commun. 2018. PMID: 30170733
Cited by
-
Examining the Potential of Vitamin C Supplementation in Tissue-Engineered Equine Superficial Digital Flexor Tendon Constructs.Int J Mol Sci. 2023 Dec 4;24(23):17098. doi: 10.3390/ijms242317098. Int J Mol Sci. 2023. PMID: 38069418 Free PMC article.
-
The Functions and Mechanisms of Tendon Stem/Progenitor Cells in Tendon Healing.Stem Cells Int. 2023 Sep 12;2023:1258024. doi: 10.1155/2023/1258024. eCollection 2023. Stem Cells Int. 2023. PMID: 37731626 Free PMC article. Review.
-
Beneficial Effects of Zoledronic Acid on Tendons of the Osteogenesis Imperfecta Mouse (Oim).Pharmaceuticals (Basel). 2023 Jun 2;16(6):832. doi: 10.3390/ph16060832. Pharmaceuticals (Basel). 2023. PMID: 37375779 Free PMC article.
-
Mechanisms and new advances in the efficacy of plant active ingredients in tendon-bone healing.J Orthop Surg Res. 2025 Jan 29;20(1):106. doi: 10.1186/s13018-025-05483-y. J Orthop Surg Res. 2025. PMID: 39881382 Free PMC article. Review.
-
Transcriptomic Analysis Provides Insights to Reveal the bmp6 Function Related to the Development of Intermuscular Bones in Zebrafish.Front Cell Dev Biol. 2022 May 12;10:821471. doi: 10.3389/fcell.2022.821471. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35646941 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

