ER-PM Contact Sites - SNARING Actors in Emerging Functions
- PMID: 33681218
- PMCID: PMC7928305
- DOI: 10.3389/fcell.2021.635518
ER-PM Contact Sites - SNARING Actors in Emerging Functions
Abstract
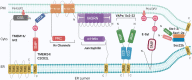
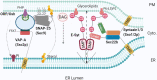
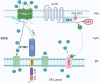
The compartmentalisation achieved by confining cytoplasm into membrane-enclosed organelles in eukaryotic cells is essential for maintaining vital functions including ATP production, synthetic and degradative pathways. While intracellular organelles are highly specialised in these functions, the restricting membranes also impede exchange of molecules responsible for the synchronised and responsive cellular activities. The initial identification of contact sites between the ER and plasma membrane (PM) provided a potential candidate structure for communication between organelles without mixing by fusion. Over the past decades, research has revealed a far broader picture of the events. Membrane contact sites (MCSs) have been recognized as increasingly important actors in cell differentiation, plasticity and maintenance, and, upon dysfunction, responsible for pathological conditions such as cancer and neurodegenerative diseases. Present in multiple organelles and cell types, MCSs promote transport of lipids and Ca2+ homoeostasis, with a range of associated protein families. Interestingly, each MCS displays a unique molecular signature, adapted to organelle functions. This review will explore the literature describing the molecular components and interactions taking place at ER-PM contact sites, their functions, and implications in eukaryotic cells, particularly neurons, with emphasis on lipid transfer proteins and emerging function of SNAREs.
Keywords: SNAREs; lipid transfer; membrane contact sites; neurons; tethers.
Copyright © 2021 Hewlett, Singh, Vannier and Galli.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

