The Interplay Between Tumor Suppressor p53 and Hypoxia Signaling Pathways in Cancer
- PMID: 33681231
- PMCID: PMC7930565
- DOI: 10.3389/fcell.2021.648808
The Interplay Between Tumor Suppressor p53 and Hypoxia Signaling Pathways in Cancer
Abstract
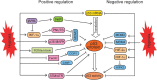
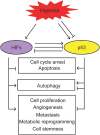
Hypoxia is a hallmark of solid tumors and plays a critical role in different steps of tumor progression, including proliferation, survival, angiogenesis, metastasis, metabolic reprogramming, and stemness of cancer cells. Activation of the hypoxia-inducible factor (HIF) signaling plays a critical role in regulating hypoxic responses in tumors. As a key tumor suppressor and transcription factor, p53 responds to a wide variety of stress signals, including hypoxia, and selectively transcribes its target genes to regulate various cellular responses to exert its function in tumor suppression. Studies have demonstrated a close but complex interplay between hypoxia and p53 signaling pathways. The p53 levels and activities can be regulated by the hypoxia and HIF signaling differently depending on the cell/tissue type and the severity and duration of hypoxia. On the other hand, p53 regulates the hypoxia and HIF signaling at multiple levels. Many tumor-associated mutant p53 proteins display gain-of-function (GOF) oncogenic activities to promote cancer progression. Emerging evidence has also shown that GOF mutant p53 can promote cancer progression through its interplay with the hypoxia and HIF signaling pathway. In this review, we summarize our current understanding of the interplay between the hypoxia and p53 signaling pathways, its impact upon cancer progression, and its potential application in cancer therapy.
Keywords: HIF; cancer; cancer therapy; hypoxia; mutant p53; p53; tumor suppressor.
Copyright © 2021 Zhang, Liu, Wang, Zhang, Xu, Hu and Feng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Amelio I., Mancini M., Petrova V., Cairns R. A., Vikhreva P., Nicolai S., et al. (2018). p53 mutants cooperate with HIF-1 in transcriptional regulation of extracellular matrix components to promote tumor progression. Proc. Natl. Acad. Sci. U.S.A. 115 E10869–E10878. 10.1073/pnas.1808314115 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

