Imidazoline Gemini Surfactants as Corrosion Inhibitors for Carbon Steel X70 in NaCl Solution
- PMID: 33681604
- PMCID: PMC7931391
- DOI: 10.1021/acsomega.0c06103
Imidazoline Gemini Surfactants as Corrosion Inhibitors for Carbon Steel X70 in NaCl Solution
Abstract
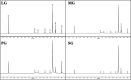
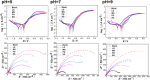
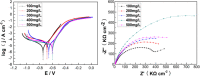
The Gemini imidazoline surfactants were synthesized from a series of saturated fatty acids. Electrochemical impedance spectroscopy (EIS), polarization curve, and quantum chemistry methods were used to study the corrosion inhibition behavior of Gemini imidazoline surfactants for X70 carbon steel in NaCl solution. Results reveal that such kind of surfactants has an outstanding inhibition effect on carbon steel X70 in NaCl solution and that the restrained efficiency of Gemini imidazoline in an alkaline solution is better than that in a neutral solution. The shorter the carbon chain length, the higher the suppressive efficiency. The higher the concentration of Gemini imidazoline surfactant, the better the inhibition effect.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Schlegel M. L.; Necib S.; Daumas S.; Labat M.; Blanc C.; Foy E.; Linard Y. Corrosion at the carbon steel-clay borehole water interface under anoxic alkaline and fluctuating temperature conditions. Corros. Sci. 2018, 136, 70–90. 10.1016/j.corsci.2018.02.052. - DOI
-
- Zhao M. C.; Yang K.; Xiao F. R.; Shan Y. Y. Continuous cooling transformation of undeformed and deformed low carbon pipeline steels. Mater. Sci. Eng., A 2003, 355, 126–136. 10.1016/S0921-5093(03)00074-1. - DOI
-
- Panossian Z.; Almeida N. L.; Sousa M. F. R.; Pimenta G. D. S.; Marques L. B. S. Corrosion of carbon steel pipes and tanks by concentrated sulfuric acid: A review. Corros. Sci. 2012, 58, 1–11. 10.1016/j.corsci.2012.01.025. - DOI
-
- Usher K. M.; Kaksonen A. H.; Cole I.; et al. Microbially influenced corrosion of buried carbon steel pipes. Int. Biodeterior. Biodegrad. 2014, 93, 84–106. 10.1016/j.ibiod.2014.05.007. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

