Porphyromonas Gingivalis May Seek the Alzheimer's Disease Brain to Acquire Iron from Its Surplus
- PMID: 33681719
- PMCID: PMC7903007
- DOI: 10.3233/ADR-200272
Porphyromonas Gingivalis May Seek the Alzheimer's Disease Brain to Acquire Iron from Its Surplus
Abstract
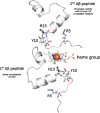
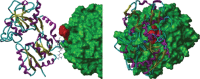
Iron accumulates in the brain of subjects with Alzheimer's disease (AD). Here it promotes the aggregation of amyloid-β plaques in which it is abundant. Iron induces amyloid-β neurotoxicity by damaging free radicals and causing oxidative stress in brain areas with neurodegeneration. It can also bind to tau in AD and enhance the toxicity of tau through co-localization with neurofibrillary tangles and induce accumulation of these tangles. Porphyromonas gingivalis is a key oral pathogen in the widespread biofilm-induced disease "chronic" periodontitis, and recently, has been suggested to have an important role in the pathogenesis of AD. P. gingivalis has an obligate requirement for iron. The current paper suggests that P. gingivalis seeks the AD brain, where it has been identified, to satisfy this need. If this is correct, iron chelators binding iron could have beneficial effects in the treatment of AD. Indeed, studies from both animal AD models and humans with AD have indicated that iron chelators, e.g., lactoferrin, can have such effects. Lactoferrin can also inhibit P. gingivalis growth and proteinases and its ability to form biofilm.
Keywords: Biofilm; brain; immunity; iron dysregulation; iron requirement; senescence.
© 2021 – The authors. Published by IOS Press.
Conflict of interest statement
The author has no conflict of interest to report.
Figures
Similar articles
-
Porphyromonas gingivalis-Induced Neuroinflammation in Alzheimer's Disease.Front Neurosci. 2021 Oct 14;15:691016. doi: 10.3389/fnins.2021.691016. eCollection 2021. Front Neurosci. 2021. PMID: 34720846 Free PMC article. Review.
-
Hypothesis: apo-lactoferrin-Galantamine Proteo-alkaloid Conjugate for Alzheimer's disease Intervention.J Cell Mol Med. 2018 Mar;22(3):1957-1963. doi: 10.1111/jcmm.13484. Epub 2018 Jan 29. J Cell Mol Med. 2018. PMID: 29377514 Free PMC article.
-
Porphyromonas gingivalis Outer Membrane Vesicles as the Major Driver of and Explanation for Neuropathogenesis, the Cholinergic Hypothesis, Iron Dyshomeostasis, and Salivary Lactoferrin in Alzheimer's Disease.J Alzheimers Dis. 2021;82(4):1417-1450. doi: 10.3233/JAD-210448. J Alzheimers Dis. 2021. PMID: 34275903 Free PMC article.
-
Porphyromonas gingivalis is a Strong Risk Factor for Alzheimer's Disease.J Alzheimers Dis Rep. 2020 Dec 14;4(1):501-511. doi: 10.3233/ADR-200250. J Alzheimers Dis Rep. 2020. PMID: 33532698 Free PMC article. Review.
-
Assessing the role of Porphyromonas gingivalis in periodontitis to determine a causative relationship with Alzheimer's disease.J Oral Microbiol. 2019 Jan 29;11(1):1563405. doi: 10.1080/20002297.2018.1563405. eCollection 2019. J Oral Microbiol. 2019. PMID: 30728914 Free PMC article. Review.
Cited by
-
The role of microbiome-host interactions in the development of Alzheimer´s disease.Front Cell Infect Microbiol. 2023 Jun 2;13:1151021. doi: 10.3389/fcimb.2023.1151021. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37333848 Free PMC article. Review.
-
Can Porphyromonas gingivalis Contribute to Alzheimer's Disease Already at the Stage of Gingivitis?J Alzheimers Dis Rep. 2021 Apr 6;5(1):237-241. doi: 10.3233/ADR-210006. J Alzheimers Dis Rep. 2021. PMID: 34113781 Free PMC article.
-
Virus-Induced Membrane Fusion in Neurodegenerative Disorders.Front Cell Infect Microbiol. 2022 Mar 24;12:845580. doi: 10.3389/fcimb.2022.845580. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35531328 Free PMC article. Review.
-
Porphyromonas gingivalis-Induced Neuroinflammation in Alzheimer's Disease.Front Neurosci. 2021 Oct 14;15:691016. doi: 10.3389/fnins.2021.691016. eCollection 2021. Front Neurosci. 2021. PMID: 34720846 Free PMC article. Review.
References
-
- Aquino D, Bizzi A, Grisoli M, Garavaglia B, Bruzzone MG, Nardocci N, Savoiardo M, Chiapparini L (2009) Age-related iron deposition in the basal ganglia: Quantitative analysis in healthy subjects. Radiology 252, 165–172. - PubMed
-
- Mantyh PW, Ghilardi JR, Rogers S, DeMaster E, Allen CJ, Stimson ER, Maggio JE (1993) Aluminum, iron, and zinc ions promote aggregation of physiological concentrations of β-amyloid peptide. J Neurochem 61, 1171–1174. - PubMed
-
- Huang X, Atwood CS, Moir RD, Hartshorn MA, Tanzi RE, Bush AI (2004) Trace metal contamination initiates the apparent auto-aggregation, amyloidosis, and oligomerization of Alzheimer’s Aβ peptides. J Biol Inorg Chem 9, 954–960. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

