Targeting androgen regulation of TMPRSS2 and ACE2 as a therapeutic strategy to combat COVID-19
- PMID: 33681723
- PMCID: PMC7919514
- DOI: 10.1016/j.isci.2021.102254
Targeting androgen regulation of TMPRSS2 and ACE2 as a therapeutic strategy to combat COVID-19
Abstract
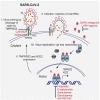
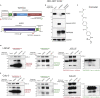
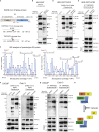
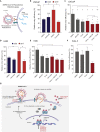
Epidemiological data showing increased severity and mortality of COVID-19 in men suggests a potential role for androgen in SARS-CoV-2 infection. Here, we present evidence for the transcriptional regulation of SARS-CoV-2 host cell receptor ACE2 and TMPRSS2 by androgen in mouse and human cells. Additionally, we demonstrate the endogenous interaction between TMPRSS2 and ACE2 in human cells and validate ACE2 as a TMPRSS2 substrate. Furthermore, camostat-a TMPRSS2 inhibitor-blocked the cleavage of pseudotype SARS-CoV-2 surface Spike without disrupting TMPRSS2-ACE2 interaction, thus providing evidence for the first time of a direct role of TMPRSS2 in priming the SARS-CoV-2 Spike, required for viral fusion to the host cell. Importantly, androgen-deprivation, anti-androgens, or camostat attenuated the SARS-CoV-2 S-mediated cellular entry. Together, our data provide a strong rationale for clinical evaluations of TMPRSS2 inhibitors and androgen-deprivation therapy/androgen receptor antagonists alone or in combination with antiviral drugs as early as clinically possible to prevent COVID-19 progression.
Keywords: Biological Sciences; Molecular Biology; Virology.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Afar D.E., Vivanco I., Hubert R.S., Kuo J., Chen E., Saffran D.C., Raitano A.B., Jakobovits A. Catalytic cleavage of the androgen-regulated TMPRSS2 protease results in its secretion by prostate and prostate cancer epithelia. Cancer Res. 2001;61:1686–1692. - PubMed
-
- de Bono J.S., Guo C., Gurel B., De Marzo A.M., Sfanos K.S., Mani R.S., Gil J., Drake C.G., Alimonti A. Prostate carcinogenesis: inflammatory storms. Nat. Rev. Cancer. 2020;20:455–469. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

