Hydroxychloroquine with or without azithromycin for treatment of early SARS-CoV-2 infection among high-risk outpatient adults: A randomized clinical trial
- PMID: 33681731
- PMCID: PMC7912360
- DOI: 10.1016/j.eclinm.2021.100773
Hydroxychloroquine with or without azithromycin for treatment of early SARS-CoV-2 infection among high-risk outpatient adults: A randomized clinical trial
Abstract
Background: Treatment options for outpatients with COVID-19 could reduce morbidity and prevent SARS-CoV-2 transmission.
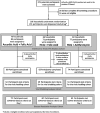
Methods: In this randomized, double-blind, three-arm (1:1:1) placebo-equivalent controlled trial conducted remotely throughout the United States, adult outpatients with laboratory-confirmed SARS-CoV-2 infection were recruited. Participants were randomly assigned to receive hydroxychloroquine (HCQ) (400 mg BID x1day, followed by 200 mg BID x9days) with or without azithromycin (AZ) (500 mg, then 250 mg daily x4days) or placebo-equivalent (ascorbic acid (HCQ) and folic acid (AZ)), stratified by risk for progression to severe COVID-19 (high-risk vs. low-risk). Self-collected nasal swabs for SARS-CoV-2 PCR, FLUPro symptom surveys, EKGs and vital signs were collected daily. Primary endpoints were: (a) 14-day progression to lower respiratory tract infection (LRTI), 28-day COVID-19 related hospitalization, or death; (b) 14-day time to viral clearance; secondary endpoints included time to symptom resolution (ClinicalTrials.gov: NCT04354428). Due to the low rate of clinical outcomes, the study was terminated for operational futility.
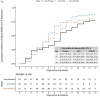
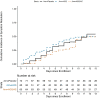
Findings: Between 15th April and 27th July 2020, 231 participants were enrolled and 219 initiated medication a median of 5.9 days after symptom onset. Among 129 high-risk participants, incident LRTI occurred in six (4.7%) participants (two control, four HCQ/AZ) and COVID-19 related hospitalization in seven (5.4%) (four control, one HCQ, two HCQ/AZ); no LRTI and two (2%) hospitalizations occurred in the 102 low-risk participants (one HCQ, one HCQ/AZ). There were no deaths. Among 152 participants with viral shedding at enrollment, median time to clearance was 5 days (95% CI=4-6) in HCQ, 6 days (95% CI=4-8) in HCQ/AZ, and 8 days (95% CI=6-10) in control. Viral clearance was faster in HCQ (HR=1.62, 95% CI=1.01-2.60, p = 0.047) but not HCQ/AZ (HR=1.25, p = 0.39) compared to control. Among 197 participants who met the COVID-19 definition at enrollment, time to symptom resolution did not differ by group (HCQ: HR=1.02, 95% CI-0.63-1.64, p = 0.95, HCQ/AZ: HR=0.91, 95% CI=0.57-1.45, p = 0.70).
Interpretation: Neither HCQ nor HCQ/AZ shortened the clinical course of outpatients with COVID-19, and HCQ, but not HCQ/AZ, had only a modest effect on SARS-CoV-2 viral shedding. HCQ and HCQ/AZ are not effective therapies for outpatient treatment of SARV-CoV-2 infection.
Funding: The COVID-19 Early Treatment Study was funded by the Bill & Melinda Gates Foundation (INV-017062) through the COVID-19 Therapeutics Accelerator. University of Washington Institute of Translational Health Science (ITHS) grant support (UL1 TR002319), KL2 TR002317, and TL1 TR002318 from NCATS/NIH funded REDCap. The content is solely the responsibility of the authors and does not necessarily represent the views, decisions, or policies of the institutions with which they are affiliated. PAN and MJA were supported by the Mayo Clinic Windland Smith Rice Comprehensive Sudden Cardiac Death Program.Trial registration ClinicalTrials.gov number NCT04354428.
Keywords: Azithromycin; COVID-19; Coronavirus; Early treatment; Hydroxychloroquine; Randomized controlled trial; Remote enrollment; SARS-CoV-2.
© 2021 The Author(s).
Figures

References
-
- Wu Z., McGoogan J.M., Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1242. - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

