The metformin in tuberous sclerosis (MiTS) study: A randomised double-blind placebo-controlled trial
- PMID: 33681737
- PMCID: PMC7910694
- DOI: 10.1016/j.eclinm.2020.100715
The metformin in tuberous sclerosis (MiTS) study: A randomised double-blind placebo-controlled trial
Abstract
Background: Tuberous Sclerosis Complex (TSC) is a genetic disorder characterised by the development of benign tumours secondary to loss of inhibitory regulation of the mTOR (mechanistic Target of Rapamycin) intracellular growth pathway. Metformin inhibits the mTOR pathway. We investigated whether metformin would reduce growth of hamartomas associated with tuberous sclerosis complex.
Methods: In this multicentre randomized, double-blind, placebo-controlled trial, patients with a clinical diagnosis of tuberous sclerosis, aged over 10 years and with at least one renal angiomyolipoma of greater than 1 cm in diameter were enrolled. Participants were randomly allocated (1:1) by a secure website to receive metformin or placebo for 12 months. The primary outcome was percentage volume change of renal angiomyolipomas (AML) at 12 months compared to baseline. Secondary outcomes were percentage change at 12 months from baseline in volume of cerebral Subependymal Giant Cell Astrocytomas (SEGA); appearance of facial and ungual hamartomas; frequency of epileptic seizures; and adaptive behaviour. The trial is registered with The International Standard Randomised Controlled Trial Number (ISRCTN), number 92545532, and the European Union Drug Regulating Authorities Clinical Trials (EUDRACT), number 2011-001319-30.
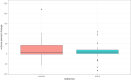
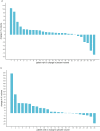
Findings: Between 1 November 2012 and 30 September 2015 72 patients were screened and 55 were randomly assigned to metformin (28) or placebo (27). Four participants withdrew between randomisation and starting treatment. All 51 patients who started therapy completed the trial and were assessed for outcome at 12 months. The median percentage change in angiomyolipoma (AML) volume was +7.6% (IQR -1.8% to +42.6%) for the placebo group and +8.9% (IQR 1.3% to 19.5%) for the metformin group (p = 0.28). Twenty-seven patients had SEGAs: 13 received placebo and 14 metformin. The median percentage change in SEGA volume was +3.0% (IQR -22.8% to +27.7%) for the placebo group and - 20.8% (IQR - 47.1% to - 5.0%) for the metformin group (p = 0.03). Twenty-one patients were assessed for seizure frequency: 9 received placebo and 12 received metformin. In the metformin group, a mean reduction of 43.7% from baseline in seizures was observed and in the placebo group a 3.1% mean reduction was observed, with a difference in response of 40.6% (95% CI -3.1% to +84.2%, p = 0.03). There were no significant differences between metformin and placebo groups for the other secondary outcomes. There were no deaths. Three serious adverse events (SAEs) occurred during the trial (all patients on metformin).
Interpretation: Metformin did not reduce AML volume. Metformin did reduce SEGA volume and seizure frequency compared with placebo. There may be a role for metformin in slowing or reversing growth of some life-threatening hamartomas in TSC and for reducing seizure frequency. Further study is justified.
Funding: This study was funded by the National Institute for Health and Research (NIHR) through the The Research for Patient Benefit Programme (RfPB).
© 2020 The Authors.
Conflict of interest statement
All other authors have no interests to declare.
Figures


Similar articles
-
Everolimus for subependymal giant cell astrocytoma in patients with tuberous sclerosis complex: 2-year open-label extension of the randomised EXIST-1 study.Lancet Oncol. 2014 Dec;15(13):1513-1520. doi: 10.1016/S1470-2045(14)70489-9. Epub 2014 Nov 10. Lancet Oncol. 2014. PMID: 25456370 Clinical Trial.
-
Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial.Lancet. 2013 Jan 12;381(9861):125-32. doi: 10.1016/S0140-6736(12)61134-9. Epub 2012 Nov 14. Lancet. 2013. PMID: 23158522 Clinical Trial.
-
Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial.Lancet. 2013 Mar 9;381(9869):817-24. doi: 10.1016/S0140-6736(12)61767-X. Lancet. 2013. PMID: 23312829 Clinical Trial.
-
Amiloride, fluoxetine or riluzole to reduce brain volume loss in secondary progressive multiple sclerosis: the MS-SMART four-arm RCT.Southampton (UK): NIHR Journals Library; 2020 May. Southampton (UK): NIHR Journals Library; 2020 May. PMID: 32453521 Free Books & Documents. Review.
-
Antidepressant treatment with sertraline for adults with depressive symptoms in primary care: the PANDA research programme including RCT.Southampton (UK): NIHR Journals Library; 2019 Dec. Southampton (UK): NIHR Journals Library; 2019 Dec. PMID: 31869013 Free Books & Documents. Review.
Cited by
-
New insights on the potential anti-epileptic effect of metformin: Mechanistic pathway.J Cell Mol Med. 2023 Dec;27(24):3953-3965. doi: 10.1111/jcmm.17965. Epub 2023 Sep 22. J Cell Mol Med. 2023. PMID: 37737447 Free PMC article. Review.
-
Therapeutic Approaches to Tuberous Sclerosis Complex: From Available Therapies to Promising Drug Targets.Biomolecules. 2024 Sep 21;14(9):1190. doi: 10.3390/biom14091190. Biomolecules. 2024. PMID: 39334956 Free PMC article. Review.
-
Epileptogenesis in tuberous sclerosis complex-related developmental and epileptic encephalopathy.Brain. 2023 Jul 3;146(7):2694-2710. doi: 10.1093/brain/awad048. Brain. 2023. PMID: 36806388 Free PMC article. Review.
-
Epilepsy and autophagy modulators: a therapeutic split.Autophagy. 2025 Sep;21(9):1863-1887. doi: 10.1080/15548627.2025.2506292. Epub 2025 May 30. Autophagy. 2025. PMID: 40375490 Free PMC article. Review.
-
mTOR pathway diseases: challenges and opportunities from bench to bedside and the mTOR node.Orphanet J Rare Dis. 2025 May 27;20(1):256. doi: 10.1186/s13023-025-03740-1. Orphanet J Rare Dis. 2025. PMID: 40426219 Free PMC article. Review.
References
-
- O'Callaghan F.J., Shiell A.W., Osborne J.P., Martyn C.N. Prevalence of tuberous sclerosis estimated by capture-recapture analysis. Lancet. 1998;351(9114):1490. - PubMed
-
- Pulsifer M.B., Winterkorn E.B., Thiele E.A. Psychological profile of adults with tuberous sclerosis complex. Epilepsy Behav. 2007;10(3):402–406. - PubMed
-
- Ferguson A.P., McKinlay I.A., Hunt A. Care of adolescents with severe learning disability from tuberous sclerosis. Dev Med Child Neurol. 2002;44(4):256–262. - PubMed
-
- van Slegtenhorst M., de Hoogt R., Hermans C., Nellist M., Janssen B., Verhoef S. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science. 1997;277(5327):805–808. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

