Adjuvant melatonin for the prevention of recurrence and mortality following lung cancer resection (AMPLCaRe): A randomized placebo controlled clinical trial
- PMID: 33681747
- PMCID: PMC7930365
- DOI: 10.1016/j.eclinm.2021.100763
Adjuvant melatonin for the prevention of recurrence and mortality following lung cancer resection (AMPLCaRe): A randomized placebo controlled clinical trial
Abstract
Background: Despite curative intent resection in patients with non-small cell lung cancer (NSCLC), recurrence leading to mortality remains too common. Melatonin has shown promise for the treatment of patients with lung cancer; however, its effect following cancer resection has not been studied. We evaluated if melatonin taken after complete resection reduces lung cancer recurrence and mortality, or impacts quality of life (QOL), symptomatology or immune function.
Methods: Participants received melatonin (20 mg) or placebo nightly for one year following surgical resection of primary NSCLC. The primary outcome was two-year disease-free survival (DFS). Secondary outcomes included five-year DFS, adverse events, QOL, fatigue, sleep, depression, anxiety, pain, and biomarkers assessing for immune function/inflammation. This study is registered at https://clinicaltrials.gov NCT00668707.
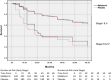
Findings: 709 patients across eight centres were randomized to melatonin (n = 356) versus placebo (n = 353). At two years, melatonin showed a relative risk of 1·01 (95% CI 0·83-1·22), p = 0·94 for DFS. At five years, melatonin showed a hazard ratio of 0·97 (95% CI 0·86-1·09), p = 0·84 for DFS. When stratified by cancer stage (I/II and III/IV), a hazard reduction of 25% (HR 0·75, 95% CI 0·61-0·92, p = 0·005) in five-year DFS was seen for participants in the treatment arm with advanced cancer (stage III/IV). No meaningful differences were seen in any other outcomes.
Interpretation: Adjuvant melatonin following resection of NSCLC does not affect DFS for patients with resected early stage NSCLC, yet may increase DFS in patients with late stage disease. Further study is needed to confirm this positive result. No beneficial effects were seen in QOL, symptoms, or immune function.
Funding: This study was funded by the Lotte and John Hecht Memorial Foundation and the Gateway for Cancer Research Foundation.
© 2021 The Authors.
Conflict of interest statement
Dr. Villeneuve reports ‘other’ from Minogue Medical, outside the submitted work. All other authors have no conflicts of interest to disclose.
Figures



References
-
- Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. - PubMed
-
- Blanchon F., Grivaux M., Asselain B., Lebas F.-.X., Orlando J.-.P., Piquet J. 4-year mortality in patients with non-small-cell lung cancer: development and validation of a prognostic index. Lancet Oncol. 2006 Oct;7(10):829–836. - PubMed
-
- Ettinger D.S., Wood D.E., Aisner D.L., Akerley W., Bauman J., Chirieac L.R. Non-small cell lung cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017 Apr;15(4):504–535. - PubMed
-
- Miller V.A. Optimizing therapy in previously treated non-small cell lung cancer. Semin Oncol. 2006 Feb;33:S25–S31. 1 Suppl 1. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

