Real-world experience with obeticholic acid in patients with primary biliary cholangitis
- PMID: 33681748
- PMCID: PMC7930359
- DOI: 10.1016/j.jhepr.2021.100248
Real-world experience with obeticholic acid in patients with primary biliary cholangitis
Abstract
Background & aims: Obeticholic acid (OCA) is the second-line treatment approved for patients with primary biliary cholangitis (PBC) and an inadequate response or intolerance to ursodeoxycholic acid. We aimed to evaluate the effectiveness and safety of OCA under real-world conditions.
Methods: Patients were recruited into the Italian PBC Registry, a multicentre, observational cohort study that monitors patients with PBC at national level. The primary endpoint was the biochemical response according to Poise criteria; the secondary endpoint was the biochemical response according to normal range criteria, defined as normal levels of bilirubin, alkaline phosphatase (ALP), and alanine aminotransferase (ALT) at 12 months. Safety and tolerability were also assessed.
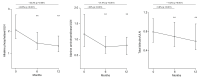
Results: We analysed 191 patients until at least 12 months of follow-up. Median age was 57 years, 94% female, 61 (32%) had cirrhosis, 28 (15%) had histologically proven overlap with autoimmune hepatitis (PBC-AIH). At 12 months, significant median reductions of ALP (-32.3%), ALT (-31.4%), and bilirubin (-11.2%) were observed. Response rates were 42.9% according to Poise criteria, and 11% by normal range criteria. Patients with cirrhosis had lower response than patients without cirrhosis (29.5% vs. 49.2%, p = 0.01), owing to a higher rate of OCA discontinuation (30% vs. 12%, p = 0.004), although with similar ALP reduction (29.4% vs. 34%, p = 0.53). Overlap PBC-AIH had a similar response to pure PBC (46.4% vs. 42.3%, p = 0.68), with higher ALT reduction at 6 months (-38% vs. -29%, p = 0.04). Thirty-three patients (17%) prematurely discontinued OCA because of adverse events, of whom 11 experienced serious adverse events. Treatment-induced pruritus was the leading cause of OCA discontinuation (67%).
Conclusions: Effectiveness and safety of OCA under real-world conditions mirror those in the Poise trial. Patients with cirrhosis had lower tolerability. Overlap PBC-AIH showed higher ALT reduction at 6 months compared with patients with pure PBC.
Lay summary: Obeticholic acid (OCA) was shown to be effective in more than one-third of patients not responding to ursodeoxycholic acid in a real-world context in Italy. Patients with cirrhosis had more side effects with OCA, and this led to suspension of the drug in one-third of patients. OCA was also effective in patients who had overlap between autoimmune hepatitis and primary biliary cholangitis.
Keywords: AIH, autoimmune hepatitis; ALP, alkaline phosphatase; ALT, alanine transferase; AMA, antimitochondrial antibodies; ANA, antinuclear antibodies; AST, aspartate transferase; Autoimmunity; CRFs, case record forms; Cholestasis; Cirrhosis; EDC, electronic data capture; GGT, gamma-glutamyl transferase; OC, Overall cohort; OCA, obeticholic acid; Overlap PBC-AIH; PBC, primary biliary cholangitis; QC, quality control; RCT, randomised controlled trial; RR, risk ratio; TCC, Treatment Completer Cohort; TIPS, transjugular intrahepatic portosystemic shunt; UDCA, ursodeoxycholic acid; ULN, upper limit of normal; aRR, adjusted risk ratio.
© 2021 The Author(s).
Conflict of interest statement
The authors have no conflicts of interest to declare related to this work. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures




References
-
- Hirschfield G.M., Beuers U., Corpechot C., Invernizzi P., Jones D.E., Marzioni M. EASL Clinical Practice Guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145–172. - PubMed
-
- Poupon R.E., Poupon R., Balkau B. Ursodiol for the long-term treatment of primary biliary cirrhosis. N Engl J Med. 1994;330:1342–1347. - PubMed
-
- Carbone M., Mells G.F., Pells G., Dawwas M.F., Newton J.L., Heneghan M.A. Sex and age are determinants of the clinical phenotype of primary biliary cirrhosis and response to ursodeoxycholic acid. Gastroenterology. 2013;144:560–569. e7. - PubMed
-
- Nevens F., Andreone P., Mazzella G., Strasser S.I., Bowlus C., Invernizzi P. A placebo-controlled trial of obeticholic acid in primary biliary cholangitis. N Engl J Med. 2016;375:631–643. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

